Chapter 2: Topography consistently drives intra- and inter-specific leaf trait variation within tree species complexes in a Neotropical forest
Accepted in Oikos with doi 10.1111/oik.07488 (Schmitt et al. 2020)
Sylvain Schmitt  ,
Bruno Hérault
,
Bruno Hérault  ,
Émilie Ducouret,
Anne Baranger,
Niklas Tysklind,
Myriam Heuertz
,
Émilie Ducouret,
Anne Baranger,
Niklas Tysklind,
Myriam Heuertz  ,
Éric Marcon
,
Éric Marcon  ,
Saint Omer Cazal,
Géraldine Derroire
,
Saint Omer Cazal,
Géraldine Derroire 
Univ. Bordeaux, INRAE, BIOGECO, 69 route d’Arcachon, 33610 Cestas France; CIRAD, UPR Forêts et Sociétés, Yamoussoukro, Côte d’Ivoire; Forêts et Sociétés, Univ Montpellier, CIRAD, Montpellier, France; Institut National Polytechnique Félix Houphouët-Boigny, INP-HB, Yamoussoukro, Côte d’Ivoire; Cirad, UMR EcoFoG (Agroparistech, CNRS, INRAE, Université des Antilles, Université de la Guyane), Campus Agronomique, 97310 Kourou, French Guiana; INRAE, UMR EcoFoG (Agroparistech, CNRS, Cirad, Université des Antilles, Université de la Guyane), Campus Agronomique, 97310 Kourou, French Guiana; UMR EcoFoG, Agroparistech, CNRS, Cirad, INRAE, Université des Antilles, Université de la Guyane, Campus Agronomique, 97310 Kourou, French Guiana
Abstract
Tropical forests shelter the highest species diversity worldwide, although genus diversity is lower than expected. In the species-rich genera, species complexes are composed of closely-related species that share large amounts of genetic variation. Despite the key role of species complexes in diversification, evolution, and functioning of ecological communities, little is known on why species complexes arise and how they are maintained in Neotropical forests. Examining how individual phenotypes vary along environmental gradients, within and among closely-related species within species complexes, can reveal processes allowing species coexistence within species complexes.
We examined leaf functional trait variation with topography in a hyperdiverse tropical forest of the Guiana Shield. We collected leaf functional traits from 766 trees belonging to five species in two species complexes in permanent plots encompassing a diversity of topographic positions. We tested the role of topography on leaf functional trait variation with a hierarchical Bayesian model, controlling for individual tree diameter effect.
We show that, mirroring what has been previously observed among species and communities, individual leaf traits covary from acquisitive to conservative strategy within species. Moreover, decreasing wetness from bottomlands to plateaus was associated with a shift of leaf traits from an acquisitive to a conservative strategy both across and within closely-related species. Our results suggest that intraspecific trait variability widens species’ niches and converges at species’ margins where niches overlap, potentially implying local neutral processes. Intraspecific trait variability favors local adaptation and divergence of closely-related species within species complexes. It is potentially maintained through interspecific sharing of genetic variation through hybridization.
Keywords
intraspecific variability; leaf traits; Paracou ; species complex; syngameon; tropical forests
Introduction
Tropical forests shelter the highest species diversity worldwide (Gaston 2000), although genus diversity is lower than expected (Cannon and Lerdau 2015). The species-rich genera can be the result of networks of partially interfertile and closely related species called syngameons (Cannon and Lerdau 2019). Indeed, interspecific hybrids occur in 16% of tree genera (Whitney et al. 2010) and many species-rich genera have higher levels of genetic polymorphism than species-poor genera, oftentimes sharing haplotypes among species, which is likely related to introgression between congeneric species (Caron et al. 2019). Syngameons respond to two contrasting evolutionary pressures, those acting at the species level maximizing local adaptation to a niche that reduces competition among species, and those acting at the syngameon level which benefit all constituent species. Syngameons as a whole might thus have a selective advantage in some cases, decreasing the overall risk of genus extinction, maximising population size, all the while increasing local adaptation of their distinct species and reducing competition among them (Cannon and Lerdau 2015).
More generally, species that share large amounts of genetic variation due to recent common ancestry and/or hybridization are defined as species complexes (Pernès and Lourd 1984). Species complexes can result from combination and reshuffling of genetic features in hybrid swarms leading to adaptive radiation and segregation among species along environmental gradients (Seehausen 2004). These processes may multiply the number of potential ecological niches, in turn reducing competition among the species and helping the species gain reproductive isolation (Runemark et al. 2019). Despite the key role of species complexes and syngameons in evolutionary processes in ecological communities (Pinheiro et al. 2018)), little is known about the reasons for their appearance and maintenance in Neotropical forests (Baraloto et al. 2012a, Steege et al. 2013, Levi et al. 2019b, Cannon and Petit 2019).
Examining individual fitness, for example through phenotypic trait variation, can shed new light on processes of species coexistence, particularly within species complexes, because changes in individual fitness may promote long-term coexistence of species (Clark 2010). For instance, variation in foliar traits is heritable and under selection, suggesting that foliar traits are linked to individual fitness (Donovan et al. 2011). Intraspecific variability can promote species coexistence when it increases niche differentiation or when it decreases fitness differences between species, thus shielding them from competitive exclusion (Turcotte and Levine 2016). This highlights the need to consider within-species variation to investigate ecological processes, for example by examining the variation in individual performance, phenotypic traits, and genes (Albert et al. 2010a, Violle et al. 2012). Phenotypic variation within species is shaped by genetic diversity in interaction with the abiotic and biotic environment, including its spatial and temporal heterogeneity (Whitlock et al. 2007), and is modulated by ontogeny (Wright and McConnaughay 2002). Describing the relative roles of these sources of variation among individuals within and among closely-related species within species complexes thus contributes to a better understanding of the ecological and evolutionary processes that underlie the maintenance of biological variation within species complexes at both levels of integration.
Functional traits reflect fundamental trade-offs determining the species’ ecological niches along environmental gradients (Wright and Westoby 2002) and shape the structure (Paine et al. 2011) and dynamics (Hérault and Piponiot 2018) of sympatric species in conjunction with their environment (Kraft et al. 2008). Functional traits have been defined as phenotypic traits that impact fitness through their effect on individual performance, which is defined as the ability to recruit, grow, survive, and reproduce in a given environment (Violle et al. 2007). Functional traits covary among species (Díaz et al. 2016) and communities (Bruelheide et al. 2018) along distinct economics spectra of leaf (Wright et al. 2004, Osnas et al. 2013, but see Lloyd et al. 2013) and wood (Chave et al. 2009). The leaf economics spectrum opposes acquisitive ecological strategies, with high photosynthetic carbon assimilation, to conservative ones with high investment in leaf defense and durability. For instance, specific leaf area (i.e. area divided by dry weight) varies among species along gradients of soil fertility and exposure to light and represents a trade-off between resource acquisition by photosynthesis and investment in leaf defense and durability (Evans and Poorter 2001a, Hodgson et al. 2011). The leaf economics spectrum is under selection in the wild and is thus partially related to individual fitness (Donovan et al. 2011). The wood economics spectrum opposes fast growing to slow growing species (Chave et al. 2009). Nevertheless, some authors advocate a unique plant economics spectrum (Reich 2014b).
Large intraspecific variability has been documented in tree allometric relationships (Vieilledent et al. 2010) and in leaf and wood trait values(Hulshof and Swenson 2010a, Messier et al. 2010b, Poorter et al. 2018), and marked differences in variability have been detected among species within communities (Siefert et al. 2015a). Several studies have investigated the role of intraspecific variability in community assembly and evidenced effects of environmental filtering (Messier et al. 2010b, Paine et al. 2011), with shifts in trait values following environmental and resource gradients (Jung et al. 2010b, Siefert and Ritchie 2016). The articulation of plant trait responses to environmental gradients at the within-species vs. the between-species levels still remains poorly investigated. (Kichenin et al. 2013) documented responses of plant functional traits to topography that were either congruent or opposed at the intra- vs. the inter-specific levels.
In the present study, we assessed variation in leaf functional trait values of individual trees within and among closely-related species within species complexes, and addressed the effects of abiotic environment on this variation. We controlled for the effect of tree diameter, as a proxy of tree size and access to light, two factors known for their effect on intraspecific trait variation (Chazdon and Kaufmann 1993, Koch et al. 2004, Woodruff et al. 2007). We measured leaf traits on a large number of individuals (766 trees > 10 cm in diameter at breast height) belonging to five Neotropical tree species from two dominant species complexes in a highly diverse tropical forest site located within the Guiana Shield in the Amazon Basin. The site encompasses a diversity of micro-habitats through hydrologic and topographic variation ranging from seasonally flooded bottomlands to drier plateaus (Ferry et al. 2010a, Allié et al. 2015). Combining tree inventories, LiDAR-derived topographic data, and leaf functional traits, we used multivariate approaches and Bayesian modelling to address the following questions: (1) how do traits covary among individuals within tree species of tropical species complexes and (2) how does the abiotic environment influence individual leaf trait values among and within closely-related species within species complexes? Based on conservation of functional strategies both within plant communities and at the global level (Díaz et al. 2016, Bruelheide et al. 2018), we expected trait covariation to be maintained at the within-species level (but see Messier et al. 2016). We hypothesized that the abiotic environment shaped trait variation both among and within species in interaction with tree diameter and access to light (Roggy et al. 2005, Coste et al. 2009).
Material and Methods
Study site
The study was conducted in the Guiana Shield, at the Paracou field station (latitude 5°18’N and longitude 52°53’W) in the coastal region of French Guiana, northern South America. The site is characterized by an average annual rainfall of 3041 mm and a mean air temperature of 25.71 °C (Aguilos et al. 2018). Old tropical forest with an exceptional richness (i.e. over 200 woody species per hectare) dwells in this lowland area characterised by heterogeneous microtopographic conditions with numerous small hills generally not exceeding 45 m a.s.l in elevation, with a dominance of Fabaceae, Chrysobalanaceae, Lecythidaceae, and Sapotaceae (Gourlet-Fleury et al. 2004).
The site comprises 16 permanent plots (fifteen 6.25 ha plots plus one 25 ha plot) which have been censused (diameter at breast height >10 cm) every 1-2 years since 1984. Trees were mapped to the nearest meter. Nine of the plots were intentionally manipulated in 1986 with a range of disturbance intensities that created a variety of biotic environments (details on the experiment in Hérault and Piponiot 2018).
Plant material
Two sampling campaigns were led in 2017 and 2018 during the dry season, between September and December. Sampling was made for two species of Symphonia (Clusiaceae) in 2017 and for three species of Eschweilera (Lecythidaceae) in 2018 (Tab. 2). All three Eschweilera species belong to the Parvifolia clade (Huang et al. 2015, Mori et al. 2016) and represent the most abundant of eleven sympatric species in the clade recorded in the 2017 inventory. The three Eschweilera species and the two Symphonia species are Amazonian hyperdominant tree species (Steege et al. 2013) and are among the most abundant species in the Paracou station (e.g. Eschweilera sagotiana was the second most abundant tree species in the 2017 inventory in Paracou).
| Genus | Species | N | TWI |
|---|---|---|---|
| Symphonia | sp.1 | 232 | 1.97 [0.97- 4.12] |
| Symphonia | globulifera | 170 | 4.24 [1.59- 6.31] |
| Eschweilera | sagotiana | 156 | 1.97 [1.06- 3.52] |
| Eschweilera | coriacea | 137 | 2.52 [0.96- 4.92] |
| Eschweilera | decolorans | 71 | 1.9 [1.06- 2.78] |
The genus Symphonia includes the well-recognised species Symphonia globulifera L.f (Clusiaceae) with two morphotypes, S. globulifera sensu stricto (S. globulifera hereafter) and Symphonia sp.1, occurring in sympatry but in differentiated habitats, with S. globulifera preferentially growing in valley bottoms and S. sp1 preferentially exploiting a variety of drier habitats. Similarly, Eschweilera sagotiana and E. coriacea have been shown to exhibit a niche differentiation for topographic position (Allié et al. 2015). The genus Symphonia and the Parvifolia clade have been both highlighted as species complexes with low phylogenetic resolution and high levels of plastid DNA sharing among sister species (Gonzalez et al. 2009, Baraloto et al. 2012a, Huang et al. 2015, Torroba-Balmori et al. 2017, Heuertz et al. 2020). Moreover, Eschweilera species share haplotypes (Caron et al. 2019) and Symphonia shows introgression between species (S. Schmitt unpublished), suggesting interspecific gene flow characteristic of syngameons.
Individual trees with diameter at breast height larger than 10 cm (DBH > 10cm) were randomly selected across all plots so as to represent the natural distribution of DBH and niche width in topographic gradients (see sample sizes in Tab. 2). For each tree five mature and healthy leaves were sampled at the top of the crown using a slingshot sampling device (BIG SHOT ® SHERILLtree), and kept in humidified ziplock bags with CO2-enriched air in darkness until measurement within the next 6 hours following a standard protocol (Pérez-Harguindeguy et al. 2013). Access to light for each sampled tree was assessed using the Dawkins index (Dawkins 1958).
Trait measurements
For each leaf, we measured five leaf functional traits that relate to resource investment strategies through light interception and carbon assimilation (Diaz et al. 1998, Wright et al. 2004), with known role in individual fitness (Donovan et al. 2011), and that can be measured on a large number of individuals (Tab. 3): leaf mass per area (i.e. leaf dry weight divided by leaf area, LMA, ), leaf dry matter content (i.e. leaf dry weight divided by fresh weight, LDMC, ), leaf thickness (LT, ), leaf area (LA, ), and leaf chlorophyll content (CC, ). The latter three traits were assessed on fresh leaves. Although LMA is a function of LDMC and LT (Vile et al. 2005), we chose to explore the distribution of the variation of the three interdependent traits within and among species, while always recognising their interrelation. The petiole was removed for all trait measurements. Leaf fresh weight was measured with an analytical balance of precision (Denver instruments, New York, USA), leaf thickness with a micrometer of precision (Motionics, Austin, USA), leaf area was quantified using the ImageJ software (Schneider et al. 2012) on scanned images of fresh leaves with a precision of , and leaf chlorophyll content was determined with a SPAD-502 instrument (Konica-Minolta, Osaka, Japan) converted with an allometric model (Coste et al. 2010). Leaf thickness and chlorophyll content measurements were repeated 3 times per leaf to account for intra-leaf variation and leaf traits were measured by a single person per genus. Leaves were then vouchered and oven-dried for at least 48 hours at 80°C before measurement of dry weight.
| Trait | Unit | Abbreviation | |||||
|---|---|---|---|---|---|---|---|
| Leaf Mass per Area | LMA | ||||||
| Leaf Dry Matter Content | LDMC | ||||||
| Leaf Thickness | LT | ||||||
| Leaf Area | LA | ||||||
| Chlorophyll Content | CC |
Phenotype descriptors
The topographic wetness index (TWI) was selected among several interdependent abiotic descriptors (supplementary material Fig. 30) as a proxy of water accumulation. TWI is defined by the cell catchment area (i.e. cumulative upslope areas draining through the cell) divided by local slope, and represents thus a relative measure of soil moisture availability (Kopecký and Čížková 2010), where a higher TWI represents a higher soil moisture availability. Waterlogging and topography have been identified as crucial for forest dynamics (Ferry et al. 2010a) and species habitat preference (Allié et al. 2015) at the Paracou study site. TWI was derived at a 1-m resolution from a 1-m resolution digital elevation model using SAGA-GIS (Conrad et al. 2015) based on a LiDAR campaign done in 2015. In order to distinguish inter- from intraspecific abiotic effects, we split TWI into and . was the mean value across all the individuals within species , and represented the interspecific abiotic effect. was the value of individual minus the mean value of species (), and represented the intraspecific abiotic effect.
The diameter at breast height (DBH, cm) was chosen to control for tree size (O’Brien et al. 1995, Zhang et al. 2004), and access to light (evidenced with Dawkins index, supplementary material Fig. 31). DBH values of sampled individuals were retrieved from the 2017 inventory of the Paracou permanent plots (supplementary material Fig. 32).
Analysis
To examine covariation across traits independently of among-species differences, we assessed covariation in leaf functional traits across individuals by within-species principal component analyses (wPCA, Dolédec and Chessel 1994) using the withinpca function in R package ade4 (Dray et al. 2007).
Then we used Bayesian modelling to test the role of topography on leaf functional trait variation among and within species. In our models, we controlled for the effect of diameter, for which an effect on intraspecific trait values through ontogeny and access to light has already been shown (Roggy et al. 2005, Coste et al. 2009, Spasojevic et al. 2014). Leaf trait () of individual () belonging to species in plot is explained by its , using an equation with a Michaelis Menten form (more likely than a linear form, Supplementary Material Tab. 9), in interaction with an additive linear form of abiotic inter- () and intraspecific () effects:
In equation (4), represents the maximum trait value expected at maximum DBH in species and is the DBH of species for which the trait reaches half of . is integrated as a random effect centered on , the mean maximum trait value expected among species, of variance . represents plot random effects (Supplementary Material Fig. 35), encompassing the potential effects of both past disturbance and microclimate (e.g. a given plot was sampled within a few days so we assumed that weather conditions were homogeneous for all sampled trees from a given plot), centered on 0 and of variance . Maximum trait value is also modulated by and the slope of TWI effects on trait values among species and within the species , respectively. Contrary to other traits, LA showed a decrease with increasing DBH so we used instead of LA to allow for a positive covariation with DBH.
Traits, DBH, and were all normalised to ease model inference and enable comparison of traits and covariates. A Bayesian method was used to build the models. We inferred parameters for each leaf trait t and each genus separately, resulting in 10 models, using stan language (Carpenter et al. 2017) and the rstan package (Stan Development Team 2018) in the R environment (R Core Team 2020).
Results
Almost seventy percent of the within-species variance in leaf trait values was explained by the first two axes of the within-species Principal Component Analysis (wPCA, Fig. 5. Globally, the wPCA highlighted the covariation of the five leaf traits on the first axis, which alone represented 46% of the total trait variation. This axis opposed large leaves (i.e. high LA) to high values for all other leaf traits: LT, LDMC, CC and LMA. Additionally, LT and LDMC were orthogonal to an axis opposing CC and LDMC to LA. As expected, we found a strong and significant correlation between LMA and the product of LT and LDMC (, ).
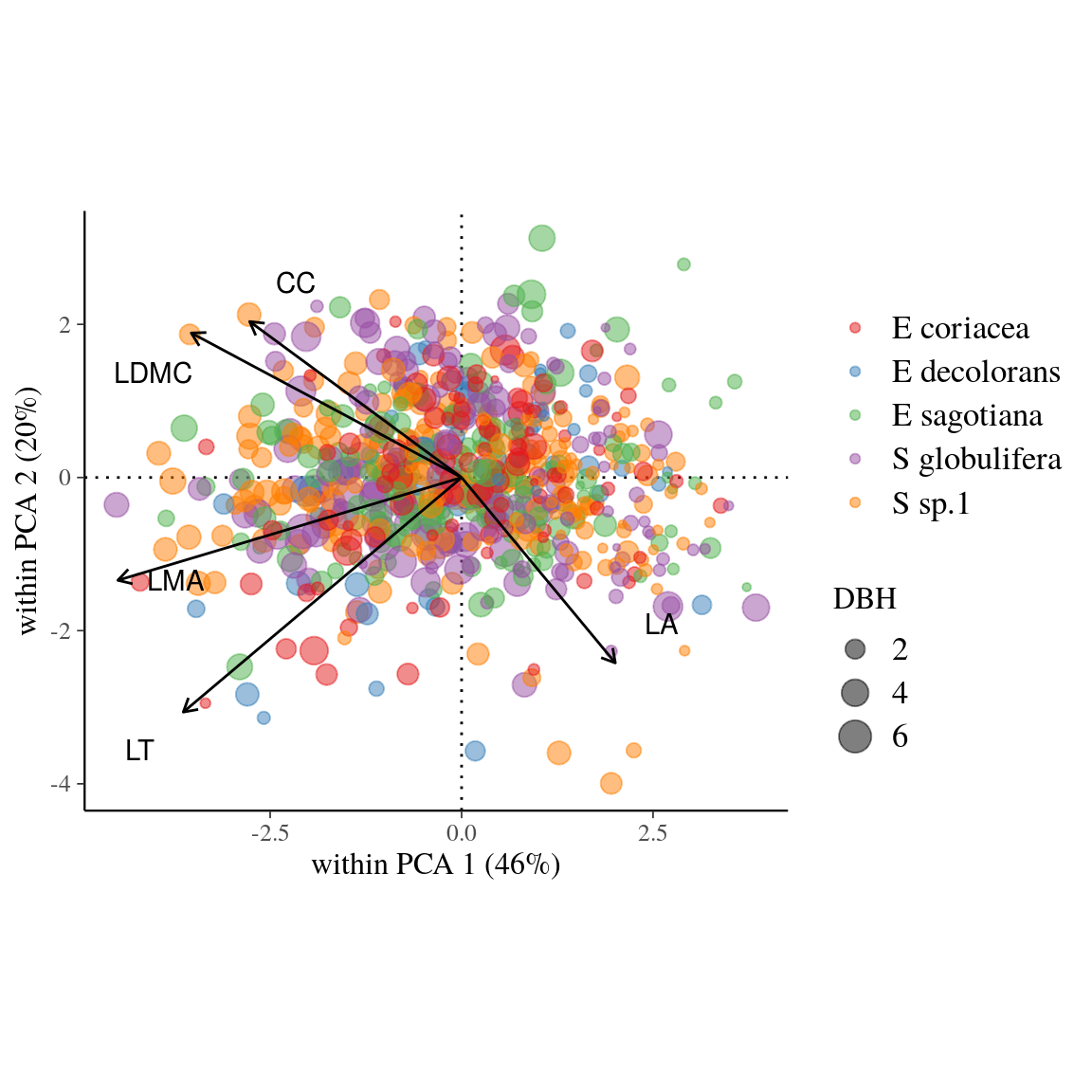
Figure 5: Within-species Principal Component Analysis (wPCA) of leaf traits across individuals in five Neotropical tree species. Circle colors indicate the species, whereas size of circles indicates individual diameter at breast height. See Tab. 3 for abbreviation of traits.
Taking into account the strong and expected effect of tree diameter (DBH, Tab. 4 and supplementary material Tab. 33), the parameterized models showed both inter- and intraspecific effect of topography (TWI) on leaf functional trait values, validating our hypothesis. The fitted models had substantial squared deviation with around 0.8 (Fig.B 4). In Symphonia, TWI had a positive effect on LA and a negative effect on LMA, LDMC, and CC both among and within species, i.e. Symphonia trees in wetter habitat had larger and lighter leaves with less chlorophyll than those in drier habitat. This result was obtained within S. globulifera and within S. sp1 and as well as S. globulifera and S. sp1 (Fig. 6 and Table 4). In Eschweilera, TWI had a positive effect on LA and CC and a negative effect on LT and LDMC both among and within species, i.e., Eschweilera trees in wetter habitat had thinner and larger leaves with higher chlorophyll content but overall lower dry matter content. For LMA, the effect of TWI was negative among Eschweilera species and within E. decolorans and E. coriacea, but weakly positive within E. sagotiana. Essentially, eight out of ten models showed congruence of inter- and intraspecific effects of TWI on leaf functional traits in the two species complexes examined, although large uncertainties were associated with many effects (especially LT in Symphonia and LMA in Eschweilera, see Figure 6). The two complexes diverged, however, in the covariation of traits: the responses of LMA, LDMC, and LA to TWI was similar for both complexes, whereas CC and LT responded in opposite ways in Symphonia vs. Eschweilera.
| Genus | Parameter | Species | |||||
|---|---|---|---|---|---|---|---|
| Eschweilera | All | 6.564 | 6.975 | 5.271 | 1.351 | 6.347 | |
| Eschweilera | E coriacea | 0.269 | 0.038 | 0.108 | 0.238 | 0.055 | |
| Eschweilera | E decolorans | 0.680 | 0.068 | 0.427 | 0.382 | 0.076 | |
| Eschweilera | E sagotiana | 0.283 | 0.069 | 0.215 | 0.934 | 0.057 | |
| Eschweilera | All | -0.678 | -0.013 | -0.585 | -0.275 | 0.091 | |
| Eschweilera | E coriacea | -0.011 | -0.035 | -0.003 | -0.020 | 0.195 | |
| Eschweilera | E decolorans | -0.162 | -0.214 | -0.108 | -0.014 | 0.259 | |
| Eschweilera | E sagotiana | 0.006 | -0.021 | -0.071 | -0.020 | 0.235 | |
| Eschweilera | All | 0.698 | 0.349 | 0.813 | 0.305 | 0.426 | |
| Eschweilera | All | 0.443 | 0.198 | 0.365 | 0.029 | 0.124 | |
| Eschweilera | All | 0.713 | 0.659 | 0.570 | 0.193 | 0.992 | |
| Eschweilera | log-likelihood | All | -61.250 | -30.319 | 17.361 | 437.283 | -176.361 |
| Symphonia | All | 5.445 | 6.260 | 4.632 | 5.627 | 7.614 | |
| Symphonia | S globulifera | 0.450 | 0.178 | 0.349 | 0.409 | 0.200 | |
| Symphonia | S sp.1 | 0.822 | 0.173 | 0.571 | 1.235 | 0.191 | |
| Symphonia | All | -0.221 | -0.181 | 0.007 | -0.812 | -0.130 | |
| Symphonia | S globulifera | -0.051 | -0.070 | 0.047 | -0.146 | -0.131 | |
| Symphonia | S sp.1 | -0.050 | -0.084 | 0.135 | -0.133 | -0.042 | |
| Symphonia | All | 0.736 | 0.759 | 0.750 | 0.771 | 0.738 | |
| Symphonia | All | 0.252 | 0.149 | 0.958 | 0.265 | 0.276 | |
| Symphonia | All | 0.753 | 0.503 | 0.644 | 0.729 | 0.900 | |
| Symphonia | log-likelihood | All | -75.262 | 92.664 | -34.137 | -63.308 | -150.485 |
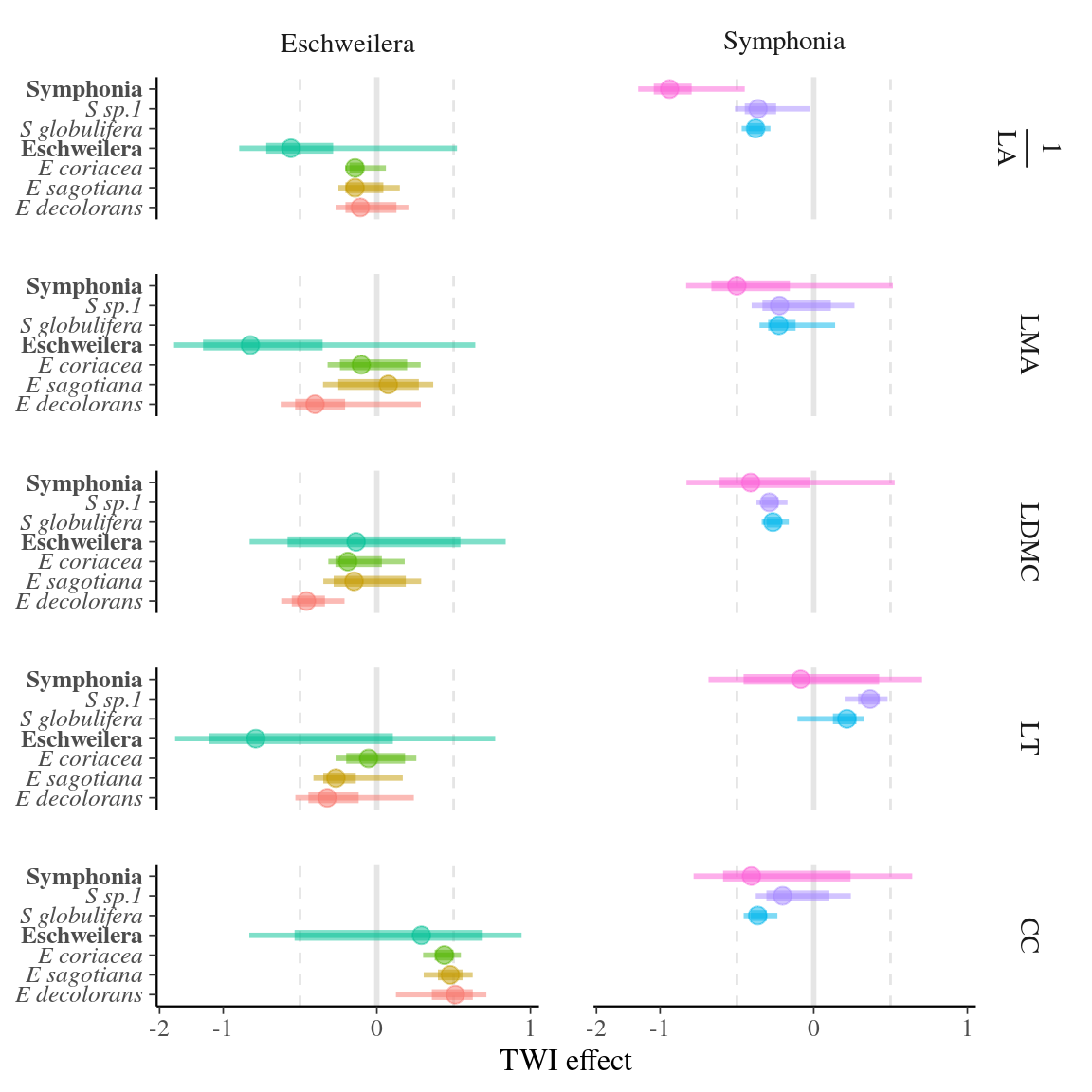
Figure 6: Inter- and intraspecific effect of topographic wetness index (TWI) on each trait for both Symphonia and Eschweilera. The effect of TWI was estimated as the posterior distribution of the slope parameters and representing respectively inter- and intraspecific effects (see Materials and Methods) using Bayesian inference. Circles represent the mean estimate, thick lines the 50% confidence interval, and thin lines the 95% confidence interval, and colour the corresponding genus for the interspecific effect or species for the intraspecific effect. See Tab. 3 for abbreviation of traits.
Discussion
Despite the key role of species complexes in Neotropical forest ecology, diversification, and evolution, little is known of the ecological drivers creating and maintaining diversity within Neotropical species complexes. Here, we show that individual leaf traits covary from acquisitive to conservative strategy within species that belong to species complexes, mirroring what has been previously observed among species and communities (Wright et al. 2004, Bruelheide et al. 2018). Specifically, decreasing water availability through higher topographic position, e.g. from bottomlands to plateaus, resulted in a shift of leaf traits from acquisitive to conservative strategies both across and within species. We discuss these results in the context of co-existence of closely related species within species complexes.
Covariation of leaf traits within species follows the leaf economics spectrum
All sampled leaf functional traits show substantial variation among individuals within species along the leaf economics spectrum (Wright et al. 2004), suggesting a conservation at the within-species level of functional strategies previously acknowledged at the among-species level (Wright et al. 2004). The first wPCA axis opposed individuals with small leaves (LA) with high thickness (LT), rich in dry matter (LDMC) and chlorophyll content (CC) and with high LMA, to individuals with large leaves (LA) with small leaf thickness (LT), poor in dry matter (LDMC) and chlorophyll content (CC) and with low LMA, within species. Covariation of LMA, LDMC, and LT across species has already been linked to a trade-off between resource acquisition by photosynthesis and investment in leaf defense and durability (Evans and Poorter 2001b, Vile et al. 2005, Baraloto et al. 2010b, Hodgson et al. 2011). In our study, LA was negatively correlated with LMA, LDMC, and LT at the intraspecific scale, whereas previous studies showed a positive correlation of LA with LMA, LT and LDMC at the interspecific scale (Fortunel et al. 2012, Poorter et al. 2018). The functional role of LA remains a subject of debate (Nicotra et al. 2011) especially regarding its contribution to common leaf functional trade-offs between species (Ackerly et al. 2002). Our results suggest that within species increasing LA contributes to a more acquisitive strategy among individuals. Individual light interception scales with both LA and the number and distribution of leaves in the crown. As individuals have more similar crown shapes within than among species, LA might be related to acquisitive strategy within species and not among species due to high variation in crown shape.
Intraspecific trait variability widens species niche
We observed a consistent response of leaf functional traits to abiotic environment both within species and among closely-related species belonging to species complexes. Topographic wetness index (TWI) characterises topographic position where water accumulates. TWI is a marker of habitat features where wetter habitats associated with increased fertility and faster demographic turnover (i.e. tree recruitment, growth, and mortality) in seasonally flooded bottomlands are opposed to nutrient-poor habitats with slower turnover at higher topographic position (Ferry et al. 2010a, Allié et al. 2015). In our study, TWI had a negative effect on LDMC and LMA, and a positive effect on LA both among and within species, i.e., sites with higher water availability were associated both with trees and species that had larger leaves with lower mass and dry matter content.
Consequently, higher topographic positioning (i.e. decreasing TWI) resulted in a shift from acquisitive to conservative functional strategy both within and among species in our study. Similar results have been found among species, where more conservative strategies were associated with drier or less fertile habitats: (i) on higher topographic position, e.g. ridge-tops (Kraft et al. 2008, Méndez-Toribio et al. 2017), (ii) with decreasing climatic water availability (Gotsch et al. 2010), and (iii) with decreased precipitation and phosphorous (Cunningham et al. 2007). Conversely, the increased fertility and water availability and faster turnover of lower elevation habitats can explain that individuals growing there show a more acquisitive strategy (Hodgson et al. 2011).
The among-species relationship between functional trait variance and the abiotic environment heterogeneity is mirrored within-species belonging to species complexes, where we find the same signatures between intraspecific trait variation and the abiotic environment (but see Kichenin et al. (2013) for opposed effects). Thus, intraspecific trait variability is widening species’ niches allowing individuals to grow in environmental conditions where their species’ average functional trait values would not have allowed them to survive (Violle et al. 2012).
Wide niches result in an increased niche overlap among closely-related species within species complexes. For instance, S. sp1 grows in drier sites, on average, than S. globulifera, but the wet limit of S. sp1 transgresses the dry limit of S. globulifera (mean and 95th percentile of TWI for S. sp1 are 1.97 and 4.12, respectively, whereas 5th percentile and mean of TWI for S. globulifera are 1.59 and 4.24, respectively, Tab. 2). Consequently, this increases functional similarity at the margin of species’ ecological niches, once individual size and species effects are accounted for (respectively, and , Tab. 4). This convergence may result from genetic exchange among sister species through hybridization specific to syngameons. If, as expected, leaf functional traits related to resource-acquisition impact individual fitness (Violle et al. 2007, Donovan et al. 2011), functional convergence at species margins may drive the fitness of individuals belonging to different species to similar values, which would support neutral coexistence processes (Hubbell 2001) between functionally-similar individuals (Hérault 2007).
Our results concerned two Amazonian basin-wide hyperdominant (Steege et al. 2013) and locally abundant species complexes. Nevertheless, other congeneric pairs of species have been shown to grow in contrasting topography and soil type (Itoh et al. 2003, Allié et al. 2015, Lan et al. 2016), and many closely-related species within abundant species complexes are segregated along topography in Paracou (Schmitt et al. in prep). In addition, studied species here encompassed a broad range of niche breadth along the topographic wetness index ( from 1.7 to 4.7, Tab. 2) close to the niche breadth observed across all species in Paracou ( from 1 to 5, Supplementary Material Fig. 34). Consequently, although our results are limited to species complexes, we expect similar patterns of individual functional response to topography within many species of the community. Indeed, other studies showed a similar shift from acquisitive to conservative functional strategies within species for grassland plants (Liancourt et al. 2013, Jung et al. 2014) and forest trees (Umaña and Swenson 2019) along topographic, drought, and elevation gradients among species not necessarily belonging to a species complex. In addition, the consistency of the functional response to topography within and between species suggests that the response is environmentally controlled, and should therefore be consistent outside of species complexes.
Within species complexes, local adaptation to fine-scale heterogeneity of abiotic environments may favor divergence of closely-related species in sympatry (Seehausen 2004). While local adaptation favors divergence, hybridization may help the transfer of genetic variability responsible for conserved leaf trait responses to topography within and among species, a hypothesis that can be tested in the future using ongoing genomic data production. Hybridization among closely-related species from species complexes has been suggested to accelerate adaptation to rapidly changing environments (Cronk and Suarez-Gonzalez 2018), and can thus contribute to increasing the adaptive potential of species in the context of growing anthropogenic disturbances (Harte et al. 2004), such as increased drought risk in the Amazon basin due to global climate change (Davidson et al. 2012).
Acknowledgements
We thank the University of Bordeaux for a PhD grant to Sylvain Schmitt and acknowledge the support of a grant from Investissement d’Avenir grants of the ANR (CEBA:ANR-10-LABX-25-01) and from the GUYAMAZON program (LECYTOMICS project). We are grateful to Pascal Petronelli and the CIRAD inventory team for their work on tree inventories and botanical identification. Special thanks go to Josselin Cazal, Ilke Gelaldi, Fabien Lehuede, Adeline Adam, Agathe Benfredj Zaleski, Numa Faucherre, and David Zipper for their assistance during sampling in Paracou station.
Data accessibility
Functional traits data have been submitted to TRY initiative (Kattge et al. 2020) under the name ParacouITV. DBH, TWI and spatial positions of individuals were extracted from the Paracou Station database, for which access is modulated by the scientific director of the station (https://paracou.cirad.fr).
References
Ackerly, D. D. et al. 2002. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: Contrasting patterns in species level and community level analyses. - Oecologia 130: 449–457.
Aguilos, M. et al. 2018. Interannual and Seasonal Variations in Ecosystem Transpiration and Water Use Efficiency in a Tropical Rainforest. - Forests 10: 14.
Albert, C. H. et al. 2010a. Intraspecific functional variability: Extent, structure and sources of variation. - Journal of Ecology 98: 604–613.
Allié, E. et al. 2015. Pervasive local-scale tree-soil habitat association in a tropical forest community. - PLoS ONE 10: e0141488.
Baraloto, C. et al. 2010b. Decoupled leaf and stem economics in rain forest trees. - Ecology Letters 13: 1338–1347.
Baraloto, C. et al. 2012a. Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities. - Journal of Ecology 100: 690–701.
Bruelheide, H. et al. 2018. Global trait–environment relationships of plant communities. - Nature Ecology & Evolution: 1.
Cannon, C. H. and Lerdau, M. 2015. Variable mating behaviors and the maintenance of tropical biodiversity. - Frontiers in Genetics 6: 183.
Cannon, C. H. and Lerdau, M. T. 2019. Demography and destiny: The syngameon in hyperdiverse systems. - Proceedings of the National Academy of Sciences: 201902040.
Cannon, C. H. and Petit, R. J. 2019. The oak syngameon: more than the sum of its parts. - New Phytologist: nph.16091.
Caron, H. et al. 2019. Chloroplast DNA variation in a hyperdiverse tropical tree community. - Ecology and Evolution 9: ece3.5096.
Carpenter, B. et al. 2017. Stan : A Probabilistic Programming Language. - Journal of Statistical Software in press.
Chave, J. et al. 2009. Towards a worldwide wood economics spectrum. - Ecology Letters 12: 351–366.
Chazdon, R. L. and Kaufmann, S. 1993. Plasticity of Leaf Anatomy of Two Rain Forest Shrubs in Relation to Photosynthetic Light Acclimation. - Functional Ecology 7: 385.
Clark, J. S. 2010. Individuals and the Variation Needed for High Species Diversity in Forest Trees. - Science 327: 1129–1132.
Conrad, O. et al. 2015. System for Automated Geoscientific Analyses (SAGA) v. 2.1.4. - Geoscientific Model Development 8: 1991–2007.
Coste, S. et al. 2009. Does ontogeny modulate irradiance-elicited plasticity of leaf traits in saplings of rain-forest tree species? A test with Dicorynia guianensis and Tachigali melinonii (Fabaceae, Caesalpinioideae). - Annals of Forest Science 66: 709–709.
Coste, S. et al. 2010. Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana. - Annals of Forest Science 67: 607–607.
Cronk, Q. C. and Suarez-Gonzalez, A. 2018. The role of interspecific hybridization in adaptive potential at range margins. - Molecular Ecology 27: 4653–4656.
Cunningham, S. A. et al. 2007. Evolutionary Divergences in Leaf Structure and Chemistry , Comparing Rainfall and Soil Nutrient Gradients. - Ecology 69: 569–588.
Davidson, E. A. et al. 2012. The Amazon basin in transition. 481: 321–328.
Dawkins, H. C. 1958. The management of natural tropical high-forests with special reference to Uganda. - Imperial Forestry Institute Paper 34: 1–155.
Diaz, S. et al. 1998. Plant functional traits and environmental filters at a regional scale. - Journal of vegetation science 9: 113–122.
Díaz, S. et al. 2016. The global spectrum of plant form and function. - Nature 529: 167–171.
Dolédec, S. and Chessel, D. 1994. Co-inertia analysis: an alternative method for studying species-environment relationships. - Freshwater Biology 31: 277–294.
Donovan, L. A. et al. 2011. The evolution of the worldwide leaf economics spectrum. - Trends in Ecology and Evolution 26: 88–95.
Dray, S. et al. 2007. The ade4 package: implementing the duality diagram for ecologists. - Journal of statistical software 22: 1–20.
Evans, J. R. and Poorter, H. 2001a. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. - Plant, Cell and Environment 24: 755–767.
Evans, J. R. and Poorter, H. 2001b. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. - Plant, Cell and Environment 24: 755–767.
Ferry, B. et al. 2010a. Higher treefall rates on slopes and waterlogged soils result in lower stand biomass and productivity in a tropical rain forest. - Journal of Ecology 98: 106–116.
Fortunel, C. et al. 2012. Leaf, stem and root tissue strategies across 758 Neotropical tree species. - Functional Ecology 26: 1153–1161.
Gaston, K. J. 2000. Global patterns in biodiversity. - Nature 405: 220–227.
Gonzalez, M. A. et al. 2009. Identification of amazonian trees with DNA barcodes. - PLoS ONE 4: e7483.
Gotsch, S. G. et al. 2010. Leaf traits and water relations of 12 evergreen species in Costa Rican wet and dry forests: Patterns of intra-specific variation across forests and seasons. - Plant Ecology 211: 133–146.
Gourlet-Fleury, S. et al. 2004. Ecology and management of a neotropical rainforest : lessons drawn from Paracou, a long-term experimental research site in French Guiana Ecology and management of a neotropical rainforest : lessons drawn from Paracou, a long-term experimental research sit. - Paris: Elsevier.
Harte, J. et al. 2004. Biodiversity conservation: climate change and extinction risk. - Nature 430: 2719.
Heuertz, M. et al. 2020. The hyperdominant tropical tree Eschweilera coriacea (Parvifolia clade, Lecythidaceae) shows higher genetic heterogeneity than sympatric Eschweilera species in French Guiana. - Plant Ecology and Evolution. 153: 67–81.
Hérault, B. 2007. Reconciling niche and neutrality through the Emergent Group approach. - Perspectives in Plant Ecology, Evolution and Systematics 9: 71–78.
Hérault, B. and Piponiot, C. 2018. Key drivers of ecosystem recovery after disturbance in a neotropical forest. - Forest Ecosystems 5: 2.
Hodgson, J. G. et al. 2011. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? - Annals of Botany 108: 1137–1345.
Huang, Y. Y. et al. 2015. Toward a phylogenetic-based generic classification of neotropical lecythidaceae—I. Status of Bertholletia, Corythophora, Eschweilera and Lecythis. - Phytotaxa 203: 085–121.
Hubbell, S. P. 2001. The unified neutral theory of biodervisity.
Hulshof, C. M. and Swenson, N. G. 2010a. Variation in leaf functional trait values within and across individuals and species: An example from a Costa Rican dry forest. - Functional Ecology 24: 217–223.
Itoh, A. et al. 2003. Importance of topography and soil texture in the spatial distribution of two sympatric dipterocarp trees in a Bornean rainforest. - Ecological Research 18: 307–320.
Jung, V. et al. 2010b. Intraspecific variability and trait-based community assembly. - Journal of ecology 98: 1134–1140.
Jung, V. et al. 2014. Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events (W Cornwell, Ed.). - Journal of Ecology 102: 45–53.
Kattge, J. et al. 2020. TRY plant trait database – enhanced coverage and open access. - Global Change Biology 26: 119–188.
Kichenin, E. et al. 2013. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient (K Kitajima, Ed.). - Functional Ecology 27: 1254–1261.
Koch, G. W. et al. 2004. The limits to tree height. - Nature 428: 851–854.
Kopecký, M. and Čížková, Š. 2010. Using topographic wetness index in vegetation ecology: Does the algorithm matter? - Applied Vegetation Science 13: 450–459.
Kraft, N. J. B. et al. 2008. Functional traits and niche-based tree community assembly in an Amazonian forest. - Science 322: 580–582.
Lan, G. et al. 2016. Species associations of congeneric species in a tropical seasonal rain forest of China. - Journal of Tropical Ecology 32: 201–212.
Levi, T. et al. 2019b. Reply to Cannon and Lerdau: Maintenance of tropical forest tree diversity. - Proceedings of the National Academy of Sciences 116: 8106–8106.
Liancourt, P. et al. 2013. Plant response to climate change varies with topography, interactions with neighbors, and ecotype. - Ecology 94: 444–453.
Lloyd, J. et al. 2013. Photosynthetically relevant foliar traits correlating better on a mass vs an area basis: of ecophysiological relevance or just a case of mathematical imperatives and statistical quicksand? - New Phytologist 199: 311–321.
Messier, J. et al. 2010b. How do traits vary across ecological scales? A case for trait-based ecology. - Ecology Letters 13: 838–848.
Messier, J. et al. 2016. Trait variation and integration across scales: is the leaf economic spectrum present at local scales? - Ecography 40: 685–697.
Méndez-Toribio, M. et al. 2017. Topographic position, but not slope aspect, drives the dominance of functional strategies of tropical dry forest trees. - Environmental Research Letters 12: 085002.
Mori, S. A. et al. 2016. Observations on the phytogeography of the LECYTHIDACEAE clade (Brazil nut family). 30: 1–85.
Nicotra, A. B. et al. 2011. The evolution and functional significance of leaf shape in the angiosperms. 38: 535–552.
O’Brien, S. T. et al. 1995. Diameter, height, crown, and age relationships in eight neotropical tree species. - Ecology 76: 1926–1939.
Osnas, J. L. D. et al. 2013. Global Leaf Trait Relationships: Mass, Area, and the Leaf Economics Spectrum. - Science 340: 741–744.
Paine, C. E. T. et al. 2011. Functional traits of individual trees reveal ecological constraints on community assembly in tropical rain forests. - Oikos 120: 720–727.
Pernès, J. and Lourd, M. 1984. Organisation des complexes d’espèces. - Gestion de ressources genetiques des plantes in press.
Pérez-Harguindeguy, N. et al. 2013. New Handbook for standardized measurment of plant functional traits worldwide. - Australian Journal of Botany 61: 167–234.
Pinheiro, F. et al. 2018. Plant Species Complexes as Models to Understand Speciation and Evolution: A Review of South American Studies. - Critical Reviews in Plant Sciences 37: 54–80.
Poorter, L. et al. 2018. Can traits predict individual growth performance? A test in a hyperdiverse tropical forest. - New Phytologist 219: 109–121.
R Core Team 2020. R: A Language and Environment for Statistical Computing.
Reich, P. B. 2014b. The world-wide ’fast-slow’ plant economics spectrum: A traits manifesto. - Journal of Ecology 102: 275–301.
Roggy, J. C. et al. 2005. Links between tree structure and functional leaf traits in the tropical forest tree Dicorynia guianensis Amshoff (Caesalpiniaceae). - Annals of Forest Science 62: 553–564.
Runemark, A. et al. 2019. Eukaryote hybrid genomes. - PLOS Genetics 15: e1008404.
Schmitt, S. et al. 2020. Topography consistently drives intra- and inter-specific leaf trait variation within tree species complexes in a Neotropical forest. - Oikos: oik.07488.
Schneider, C. A. et al. 2012. NIH Image to ImageJ: 25 years of image analysis. 9: 671–675.
Seehausen, O. 2004. Hybridization and adaptive radiation. - Trends in Ecology & Evolution 19: 198–207.
Siefert, A. and Ritchie, M. E. 2016. Intraspecific trait variation drives functional responses of old-field plant communities to nutrient enrichment. - Oecologia 181: 245–255.
Siefert, A. et al. 2015a. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. - Ecology Letters 18: 1406–1419.
Spasojevic, M. J. et al. 2014. Ontogenetic trait variation influences tree community assembly across environmental gradients. - Ecosphere 5: 1–20.
Stan Development Team 2018. Rstan: the R interface to Stan.
Steege, H. ter et al. 2013. Hyperdominance in the Amazonian Tree Flora. - Science 342: 1243092–1243092.
Torroba-Balmori, P. et al. 2017. Altitudinal gradients, biogeographic history and microhabitat adaptation affect fine-scale spatial genetic structure in African and Neotropical populations of an ancient tropical tree species. - PLOS ONE 12: e0182515.
Turcotte, M. M. and Levine, J. M. 2016. Phenotypic Plasticity and Species Coexistence. - Trends in Ecology & Evolution 31: 803–813.
Umaña, M. N. and Swenson, N. G. 2019. Intraspecific variation in traits and tree growth along an elevational gradient in a subtropical forest. - Oecologia 191: 153–164.
Vieilledent, G. et al. 2010. Individual variability in tree allometry determines light resource allocation in forest ecosystems: A hierarchical Bayesian approach. - Oecologia 163: 759–773.
Vile, D. et al. 2005. Specific leaf area and dry matter content estimate thickness in laminar leaves. - Annals of Botany 96: 1129–1136.
Violle, C. et al. 2007. Let the concept of trait be functional! - Oikos 116: 882–892.
Violle, C. et al. 2012. The return of the variance: Intraspecific variability in community ecology. - Trends in Ecology and Evolution 27: 244–252.
Whitlock, R. et al. 2007. The role of genotypic diversity in determining grassland community structure under constant environmental conditions. - Journal of Ecology 95: 895–907.
Whitney, K. D. et al. 2010. Patterns of hybridization in plants. - Perspectives in Plant Ecology, Evolution and Systematics 12: 175–182.
Woodruff, D. R. et al. 2007. Impacts of tree height on leaf hydraulic architecture and stomatal control in Douglas-fir. - Plant, Cell and Environment 30: 559–569.
Wright, I. J. and Westoby, M. 2002. Leaves at low versus high rainfall: Coordination of structure, lifespan and physiology. - New Phytologist 155: 403–416.
Wright, S. D. and McConnaughay, K. D. 2002. Interpreting phenotypic plasticity: The importance of ontogeny. 17: 119–131.
Wright, I. J. et al. 2004. The worldwide leaf economics spectrum. - Nature 428: 821–827.
Zhang, L. et al. 2004. Modeling spatial variation in tree diameter-height relationships. - Forest Ecology and Management 189: 317–329.