Discussion
Despite the key role of species complexes in Neotropical forest ecology, diversification, and evolution, little is known of the eco-evolutionary forces creating and maintaining diversity within Neotropical species complexes. Here, we showed that the species complexes of Neotropical trees cover most local gradients of topography and competition and are therefore widespread in the study site whereas most of the species within them exhibit pervasive niche differentiation along these same gradients. Specifically, in the species complexes Symphonia and Eschweilera clade Parvifolia, the decrease in water availability due to higher topographic position, e.g., from bottomlands to plateaus, has led to a change in leaf functional traits from acquisitive strategies to conservative strategies, both among and within species. Symphonia species are genetically adapted to the distribution of water and nutrients, hence they coexist locally through exploiting a broad gradient of local habitats. Conversely, Eschweilera species are differentially adapted to soil chemistry and avoid the wettest, hydromorphic habitats. Last but not least, individual tree genotypes of Symphonia species are differentially adapted to regenerate and thrive in response to the fine spatio-temporal dynamics of forest gaps with divergent adaptive growth strategies along successional niches. Consequently, topography and the dynamics of forest gaps drive fine-scale spatio-temporal adaptations of individuals within and among distinct but genetically connected species within the species complexes Symphonia globulifera and Eschweilera clade Parvifolia. Fine-scale topography drives genetic divergence and niche differentiation with genetic adaptations among species; while forest gap dynamics maintains genetic diversity with variable adaptive strategies within species, at least for Symphonia species. I suggest that adaptations of tree species and individuals to topography and dynamics of forest gaps promote coexistence within and among species within species complexes, and perhaps among forest tree species outside species complexes. I further stress the potential role of the eco-evolutionary structure of Neotropical tree syngameons in their local and regional success, and their potential role in shaping the exceptional diversity of Neotropical forests. I discuss the limitations of the low-cost capture approach to study the evolution of non-model species complexes in nature. I further emphasize the need to merge ecology and evolution in an interdisciplinary approach and I present an exploration of future simulation approaches to address the conditions of coexistence of tree species given the joint effects of topography and forest gap dynamics. Overall, I defend the primordial role of individuals within species in tropical forest diversity, suggesting that we should develop a theory of community ecology starting with individuals, because interactions with environments happen after all at the individual level.
Topography and forest gap dynamics drive fine-scale spatio-temporal adaptations of individuals within and among species from two Neotropical tree species complexes.
Symphonia globulifera and Eschweilera clade Parvifolia include distinct but connected species
We identified six distinct species in the two species complexes based on differences in morphologies, gene pools (chapter 3) and topographic niches (chapter 1), with overlapping but different functional traits (chapter 2) and growth trajectories (Fig. 18); which were maintained despite the existence of an interspecific gene flow with hybridization (chapter 3). Genetic analyses of population structure revealed the existence of three species growing in sympatry within the Symphonia globulifera species sensu lato (Fig. 8a), previously recognized as structured in two morphotypes, S. globulifera sensu stricto and S. sp.1 (Sabatier et al. 1997, Molino and Sabatier 2001, Baraloto et al. 2007). Similarly, genetic analyses of population structure revealed the existence of at least three abundant species of Eschweilera clade Parvifolia (Fig. 9), corresponding to the botanical species Eschweilera coriacea, E. sagotiana, and E. decolorans. A lack of power in genomics data prevented us from detecting less abundant species of Eschweilera clade Parvifolia. Species from the two species complexes showed distinct morphotypes (Fig. 8b), despite blurred limits due to intermediate morphologies and ontogenetic effects on morphology. Species showed pervasive niche differentiation along topography and to a lesser extent with competition (Fig. 4 and Fig. 11), notably along a variation in the distribution of water and nutrients with topographic wetness index and relative elevation (Fig. 10). Niche differentiation between species is associated with a differentiation of functional traits (Fig. 17) and growth trajectories (Fig. 18). Nevertheless, the overlap between species niches, functional traits and growth trajectories emphasizes the importance of individual variation within closely-related species within the two species complexes. Finally, genetic analyses of population structure revealed interspecific gene flow with high levels of hybridization among Symphonia species (around 5%, Fig. 43). A lack of power in genomics data prevented us from exploring gene flow in Eschweilera clade Parvifolia, but previous studies already suggested interspecific hybridization (Caron et al. 2019, Heuertz et al. 2020).
The history of Symphonia species in the Neotropics might be more complex than previously thought
Symphonia globulifera colonized the Americas from Africa ca 18-16 Ma (Dick et al. 2004). Despite morphological variation and genetic differentiation observed across 13 populations from Costa Rica, Panama, Ecuador, and French Guiana, S. globulifera was never split into more than one Neotropical species (Dick and Heuertz 2008). Our results are mainly limited to the local scale (chapter 3), and further studies are needed on a broader regional scale, but they do support the definition of three distinct Neotropical taxonomic species of Symphonia. Site frequency spectra (SFS, Fig. 19) revealed a population in expansion with an excess of rare alleles in each population, as suggested in a previous study involving a population from the Guiana Shield and on from the Western Amazon (Dick and Heuertz 2008), although Barthe et al. (2017) concluded on population demographic stability in the two Symphonia morphotypes in French Guiana based on genetic data.
Moreover, genotyped outgroups of Symphonia globulifera were used to build a pantropical phylogeny of Symphonia populations using treemix (Pickrell and Pritchard 2012).
The phylogeny revealed a high likelihood of at least one migration event between S. sp.1 and the two S. globulifera populations in Paracou (20).
The phylogeny was in agreement with a previous topology (S.C. González-Martínez pers. com.) with an ancestral population in Madagascar, and Sao Tome as the intermediate population between African and American populations.
Interestingly, in our phylogeny, S. sp1 was closer to the population of Brazil and the two S. globulifera populations were closer to the population of Costa Rica.
Our results suggest a possible more complex biogeographic history of Symphonia globulifera in the Neotropics,
but they call for further analyses before extrapolating.
Ontogeny and plasticity can strongly impede the use of leaf functional traits
I used genetic species identity, individual kinship, individual diameter at breast height, and plot identification to estimate genetic variance decomposition associated with leaf functional traits (Fig. 21). Most of the trait variation was residual, associated with tree size, or with weather (illustrated in part by plot effect, see chapter 2 material and methods). Species identity had a strong effect only on leaf area, used to determine species in the field. Genotype effect was always null or non significant. The measured leaf functional traits were thus mainly plastic and lacked a clear adaptive signature. Consequently, ontogeny, access to light, topography and weather (Fig. 23, Schmitt et al. in prep) were the main drivers of the functional trait variation measured on the sampled leaves. A strong intra-individual variation in functional traits has already been highlighted previously, for leaf thickness, transfusion tissue, and mass per area with leaf height within the tree (Koch et al. 2004, Oldham et al. 2010), and on leaf vein density, stomatal pore index and hydraulic conductance with shoot length (Leigh et al. 2011). Moreover, leaf functional traits had no or non-significant effects on individual growth potential (Fig. 22), as already evidenced in the literature (Umaña and Swenson 2019).
Functional traits have been defined as traits which impact fitness indirectly through their effect on performance (Violle et al. 2007). But my results do not suggest an adaptive signal of leaf functional traits, questioning their effect on fitness, nor an effect of leaf functional traits on individual growth, questioning their effect on individual performance. This observation underlines the need to control variation in leaf traits, including sampling date, leaf position in the crown, and ontogeny variation in order to collect trait values that would represent the typical leaf functional strategy of an individual. But is there a typical leaf functional strategy at the individual level? Leaf functional traits may be mainly plastic and wide proxies under environmental control, not representative of individual functions nor associated individual eco-evolutionary strategies, but may rather only represent the functioning of a few specific leaves within an individual.
Nevertheless, my sampling within species can guide further sampling among species. Resampling 5 mature individuals with a DBH > 30 cm, as advised in a standard protocol (Pérez-Harguindeguy et al. 2013), I was able to show that estimation of the species mean trait value can vary from 5 to 15% percent (Fig. 24); while the increase in the number of individuals sampled to 10 almost reduced the variation from the estimate of the mean value of the species’ traits by two folds. In addition, improving ontogeny and habitat control in the sampling help better estimate species mean trait values. I also advocate for an accurate sampling of individual leaves within the tree in future trait-based study of intraspecific variation, controlling for instance for the architectural development stage and the position of the sampled branch and leaf, despite the tremendous work it represents.
Topography drives species niche divergence
Pervasive niche differentiation along topography (Fig. 4) was driven by genetic divergence and adaptation (Fig. 11) within and among closely-related species within species complexes. Symphonia species specifically displayed genomic signatures of adaptations to water gradients along topography (chapter 3). In detail, species are adapted to the distribution of water and nutrients captured through the topographic wetness index and the relative elevation. These results comfort the numerous examples of niche differentiation among closely-related species growing in sympatry, along fine-scale topography (Gunatilleke et al. 2006, Engelbrecht et al. 2007, Kraft et al. 2008, Allié et al. 2015), and reveal possible adaptive forces at play behind these examples. Adaptive radiations demonstrated along steep gradients in tree caldes (Fine et al. 2004, Paun et al. 2016) could thus occur also at a fine spatial scale with gradual habitat variation.
Forest gap dynamics maintains individual genetic diversity within species
Symphonia species can grow in a diversity of successional niches with genotypic adaptive growth strategies to the fine spatio-temporal dynamics of forest gaps (Fig. 14). Fast-growing genotypes grow in forest gaps, in a bright environment with low competition, and slow-growing genotypes grow in shaded closed canopies, with little access to light and high competition. The fine spatio-temporal dynamics of forest gaps thus contributes to promoting genetic diversity within tropical tree species (chapter 4).
Eschweilera clade Parvifolia species showed also genotypic adaptations to individual neighbourhood crowding, an indirect measure of competition for light during the last three decades, and to individual growth potential (Fig. 25). But, I did not find a significant association between genotypic values for neighbourhood crowding and those for individual growth potential, thus unlike in Symphonia, I did not find evidence for growth strategies matching successional niches. This lack of evidence could be due to methodological issues or to the ecology of Eschweilera species. After filtering the genomic data, less than two thirds of the sampled individuals were maintained in the data set and these showed a particular spatial structuring with neighbourhood crowding (absence of positive autocorrelation on a short scale), which the repeated random sampling of the same number of Eschweilera individuals in Paracou did not reproduce. In addition, the lack of individuals and the poor resolution of genomic data may have added noise and brought a lack of statistical power. But, the ecology of Eschweilera is particular compared to that of Symphonia with a very slow growth varying little from one individual to another on a short time scale (Fig. 18). Thus, Eschweilera could be following a different strategy in response to forest gap dynamics, through the specialization for a specific successional niche. Or individual age is less related to diameter in Eschweilera than Symphonia due to slow growth, and may result in a greater decoupling of the current successional niche from the successional niche at the time of recruitment of the individual.
Do adaptations of tree species and individuals to topography and forest gap dynamics promote coexistence within and among species within species complexes ?
Significance of species complexes and syngameons in Neotropical forests
We tried to identify species complexes in the Paracou community based on evidence for low (phylo)-genetic resolution or plastid DNA sharing among species (chapter 1). We identified five species complexes belonging to Lecythidaceae, Chrysobalanaceae, Myristicaceae, Clusiaceae, and Sapindaceae (Gonzalez et al. 2009, Baraloto et al. 2012a, Huang et al. 2015, Torroba-Balmori et al. 2017, Caron et al. 2019, Heuertz et al. 2020), which illustrates the phylogenetic diversity of species complexes in the Angiosperms. But, all tropical tree species, genera, and families do not have numerous genetic resources and detailed phylogenies available. Detailed genetic studies often detect cryptic species in widespread and abundant, supposedly well-known, tropical tree species, e.g. in Symphonia in chapter 3 or in three genera from the African flora (Ewédjè et al. 2020). Our knowledge on the frequency and abundance of tree species complexes in the Neotropics is thus limited. However, the identified species complexes include species that are locally abundant and hyperdominant at the regional level (Steege et al. 2013), illustrating their significant role in the Neotropics.
The eco-evolutionary roles of species complexes and syngameons in the tropics are subject to debate (Cannon and Lerdau 2019, Levi et al. 2019a, b), despite their recognized importance in South America (Pinheiro et al. 2018). Levi et al. (2019a) suggested that Janzen-Connell effects, in which natural enemies restrict the recruitment near conspecific adults, were sufficient to explain the maintenance of tropical biodiversity. Cannon and Lerdau (2019) responded by underlining the possible neglected role of syngameons to account for processes left out by Levi et al. (2019a), such as the effect of demographic processes on species survival or the phylogenetic distribution of diversity. Interestingly, Levi et al. (2019b) point to the lack of literature on syngameons in tropical forests while recognizing their potential importance in maintaining biodiversity: “virtually the entire literature on hybridization between tree species is based on temperate models, often at range boundaries or on steep environmental gradients”. Indeed, knowledge on syngameons is mainly concentrated on temperate forests, and even historically on oaks (Cannon and Petit 2019). However, chapters 1 to 3, together with previous literature (Pinheiro et al. 2018), confirm the presence of syngameons in the Neotropics along subtle gradients, and a few studies in the Paleotropics show or suggest syngaemons in plants (Caujapé-Castells et al. 2017), fishes (Seehausen 2006) and even the genus Homo (Holliday 2006). Absence of proof is not proof of absence: our results contribute to answering the assertion of Levi et al. (2019b).
Tree species complexes distributed along large habitat gradients include species with pervasive niche differentiation
Fine-scale topographic variations drive differential adaptations of closely-related species within tree species complexes. Topography promotes the coexistence of closely-related species through niche differentiation within tree species complexes. Our genomic analyses were limited to Symphonia and Eschweilera clade Parvifolia, but we found similar niche partitioning in other species complexes from Paracou, which suggests similar processes in other clades. Indeed, chapters 1 and 3 suggest that the evolutionary response is environmentally controlled, and should therefore operate also outside of the studied species complexes. Pervasive effects of local topography observed pantropically (Itoh et al. 2003, Kraft et al. 2008, Ferry et al. 2010a, Allié et al. 2015, Lan et al. 2016), and related nutrients and water distribution (Baltzer et al. 2005, Baldeck et al. 2013a), could drive differential adaptations between observed closely-related species, promoting their local coexistence. Adaptive divergence could evolve both in species complexes or in more differentiated sister species. Sufficient adaptive divergence seems especially important for the coexistence of weakly differentiated species in species complexes (Tobias et al. 2014), since phylogenetic proximity and incomplete reproductive isolation increase the risk of hybridization and consequent breakdown of inter-specific adaptive divergences. On the other hand, more differentiated sister species could also coexist through emerging neutrality, e.g. by participating in a distinct group of species that are functionally similar and have similar fitness in a given environment (Scheffer and Van Nes 2006).
Forest gap dynamics, also identified as a driver of niche differentiation among closely-related tree species (Yamasaki et al. 2013), could drive differential adaptations among species in addition to its effect on within-species variation. Chapter 4 suggested a limited role of the successional niche on the divergence of Symphonia species (6%, Fig. 12). But we found the three Symphonia species to grow across a wide variety of successional niches (Fig. 15); whereas species exploiting a specific niche in the succession, such as pioneers species, could show more specific adaptations, and thus higher adaptive divergence from related species specialized on other successional niches. Similarly, abundant species with a wide topographic niche should have within-species genotypic adaptations. Indeed, chapter 3 showed important genotypic variation related to topography within species of Eschweilera clade Parvifolia (Fig. 11). Consequently, abiotic environments with topography and biotic environments with forest gap dynamics are both driving adaptations and genetic diversity within and among tree species.
Does the eco-evolutionary structure of Neotropical tree syngameons explain their local and regional success?
The local coexistence of closely-related species is governed by ecological and evolutionary processes, contingent on the history of speciation and niche differentiation (Weber and Strauss 2016). I focused on the competition for resources and the resulting niche differentiation in the previous paragraphs (Chesson 2000a, Turcotte and Levine 2016), but reproductive isolation is primordial to explain the coexistence of closely-related species in sympatry (Weber and Strauss 2016). Species must have evolved sufficient reproductive isolation to avoid the break-down of differences and their genetic homogenization through reproductive interference (Levin et al. 1996, Taylor et al. 2006, Abbott et al. 2013). Differences in phenology, e.g. flowering (Gentry 1974), or pollen dispersal, e.g. pollinators (Kay 2006), can increase reproductive isolation.
The way we perceive the role of genetic connectivity among species is changing. Despite the homogenizing power of gene flow, species-specific adaptations may be maintained or even maximised despite high levels of gene flow, especially if selective pressures are spatially and/or temporally variable (Tigano and Friesen 2016). Species adaptations and partial reproductive isolation can be maintained despite gene flow through: (i) linkage with an already diverged locus, (ii) increased resistance to gene flow following secondary contact, (iii) competition among genomic architectures, e.g. reducing recombination (Tigano and Friesen 2016). Consequently, adaptive introgression using gene flow among species diverging along the environment may imply species coevolution, or evolutionary mutualism, at the syngameon level (Cannon and Petit 2019). Gene flow among species can insure reproductive assurance by hybridization, lowering the risk of local extinction, and allow species to share innovation that benefits the whole syngameon (Cannon and Lerdau 2015). This subtle equilibrium between species adaptations and gene flow may be a powerful eco-evolutionary strategy leading to local and regional success of tropical tree syngameons (Symphonia and Eschweilera include Amazonian hyperdominant tree species (Steege et al. 2013) and some of the most abundant species in Paracou).
Do tree syngameons shape the exceptional diversity of Neotropical forests?
We found topography and forest gap dynamics to drive diversity within and among tree species within species complexes promoting their coexistence. I hypothesized the syngameon to be a subtle and fragile but evolutionary successful stage, which raises the question of the temporal dynamics of syngameons. Syngameons may not be restricted to transitions toward complete speciation of the species composing it, but syngameons may represent a temporally stable stage (Cannon and Petit 2019), with species interbreeding stably over time or with syngameons that are stable over time but changing in species composition, with some species reaching complete speciation while others emerge. Reproductive isolation, although partial, can evolve very slowly to complete isolation with little selection against hybridization, or hybridization could be favored during critical environmental disturbances. If it represents a temporally stable stage, the Symphonia syngameon shows considerable gene flow (chapter 3) despite a stable environment, which argues for the hypothesis of a slow evolution towards complete isolation. Anyway, as a temporally stable stage or as a transitional stage toward complete speciation, syngameons seem to represent a successful evolutionary strategy promoting the coexistence of sympatric closely-related species. Consequently, syngameons, and more generally species complexes, could play a key role in creating and maintaining the tremendous diversity of tropical forests by promoting the coexistence of phylogenetically and functionally similar species (Cannon and Lerdau 2019). Syngameons could participate generating biodiversity, i.e., in the ‘cradle’ (Eiserhardt et al. 2017) of the tropical forest, with their speciation through complete reproductive isolation , or they could preserve biodiversity as a tropical forest ‘museum’ (Eiserhardt et al. 2017) with a temporal stability that reduces the risk of local extinction.
Beyond species complexes: do adaptations to topography and forest gap dynamics promote coexistence within and among tree species from mature forests ?
Importance of intraspecific trait variation in the forest community
Chapter 2 revealed a consistent response of leaf functional traits to topography within and between tree species, suggesting an environmental control of leaf functional strategies. Topography similarly affects functional traits in species complexes and in fully differentiated species, so the functional response to topography within and between species should apply also outside species complexes. The only limit to a differential functional response would be a specific role of shared genetic variation through gene flow or the recent common ancestry, to conserve a similar functional strategy in species complexes but not in fully differentiated species. Indeed, once isolated, species may lose genetic diversity and individual plasticity. But the absence of effect of genotypes on functional traits (Fig. 21) strongly questions the role of shared genetic variation in the consistent functional response, unless observed trait plasticity is genetically promoted (Ghalambor et al. 2007) through recent divergence or inter-specific gene flow. Therefore, I hypothesize that environmental gradients, such as topography and associated nutrient and water distributions (Chapter 3), have a consistent effect on the variability of functional traits within and between species, widening their niches and leading to an emerging neutrality in their overlap (Chapter 2) for many species in the forest community.
Fine-scale topography is a powerful driver of tree species diversity that promotes species coexistence in tropical forests
Chapters 1 to 3 revealed the effect of gradual habitat variation through fine-scale topography on ecological divergence of species with differentiation of niches and phenotypes. But evolutionary history behind adaptive radiations falls within a continuum from sympatric ecological speciation to secondary contacts of species ecologically specialised in allopatry or parapatry (Rundell and Price 2009). Pairwise site frequency spectrum of Symphonia species (Fig. 19) did not help decipher between sympatric speciation or character displacement after a secondary contact. Demographic history modelling is still needed to establish the history of the evolution of Symphonia (Hoban et al. 2012), but a broader regional sampling may be necessary for a better understanding. In both cases, it can be assumed that the extreme competition between species in hyperdiverse communities pushes species towards fine-scale habitat specialization along stable spatio-temporal environmental gradients. Topography has been highlighted to drive differences among species at early plant life stages, which contribute to the maintenance of tropical forest diversity (Metz 2012). On the other hand, spatio-temporally unstable forest gap dynamics contribute only a little to differences between species (Chapter 4). Hence, fine-scale topography can locally represent a “species pump” in tropical forests, i.e. a cradle for species, such as the Andes on a regional scale (Mutke et al. 2014).
The studied species showed large topographic niches with areas of overlap in the Paracou community (chapter 2), which implies that neutral processes, by decreasing differences in fitness, also favour the coexistence of species via a jack-of-all-trades strategy (Turcotte and Levine 2016). Topography could thus also give rise to “superspecies”, i.e. allopatric taxa that are known or thought to have evolved to the species level (Amadon 1966).
Forest gap dynamics are a powerful driver of individual diversity in tropical forests that promotes species coexistence
Chapter 4 showed for the first time that forest gap dynamics drives individual diversity and growth adaptations within Symphonia species. Genetic adequacy with forest gap dynamics determines which individuals are successful in reaching the canopy through divergent growth strategies, with hares in forest gaps and turtles in more shaded closed-canopy patches. Because of the similar broad successional niches observed in many other species of the Paracou community (Fig. 15), we hypothesized the process underlying genotypic adaptations not to be specific to Symphonia or species complexes, but to be conserved in many tropical forest tree species, probably thanks to physiological or adaptive constraints (Ellner and Hairston Jnr 1994). Nevertheless, phenotypic plasticity remains a major process that may explain large successional niches of species (Goulet and Bellefleur 1986, Chevin et al. 2010, Gao et al. 2018) that we cannot rule out until further research is carried out. In both cases, large successional niches can promote species coexistence through emergent neutrality (Scheffer and Van Nes 2006), although forest gap dynamics also select for species with narrow successional niches such as pioneer species (Dalling and Hubbell 2002). Interestingly, Bazzaz and Carlson (1982) found late-successional species to be less plastic to light access than pioneer species, and Zangerl and Bazzaz (1983) revealed more adaptation to successional niches among genotypes in late-successional than in pioneer species. Indeed, late-successional species showed the widest breadth of traits and grew in more heterogeneous environments in space and time than pioneers in forests in Venezuela (Kammesheidt 2000). Together with the results of chapter 4, these studies argue in favour of forest gap dynamics driving genotypic adaptations with divergent growth strategies in species previously called “late-successional”, which in fact encounter a diversity of light environments and competition as seedlings.
In order to study treefall conditions, I examined the local basal area distribution prior to basal area loss, i.e. the successional stage of the forest patch before a treefall occurs. Unfortunately, studying treefalls ideally requires both large spatial and temporal coverage due to the episodic nature of treefalls (Chambers et al. 2013). Despite the huge amount of work behind the monitoring of dynamics of the forest gaps at Paracou (Hérault and Piponiot 2018), I was not able to obtain a clear pattern. Although most treefalls seem to occur when the local basal area is high, e.g. at the end of forest succession, also called climax, my results could not rule out the existence of treefalls occurring at a low local basal area during the early stages of succession (Fig. 26). These early-successional treefalls could be due to stochasticity, but a bold hypothesis would be the existence of niche-hiking pioneer species that create these early-successional treefalls. Niche-hiking results from genotypes favouring local niche construction (Schwilk and Kerr 2002), such as flammability favouring open spaces after a fire for the offspring of fire-adapted trees. In the case of treefall, one could imagine the existence of an adaptation to falls for genotypes or species that grow in forest gaps. Indeed, one could imagine fast-growing genotypes or species in forest gaps are mechanically more prone to fall once in the canopy. But, niche-hiking in forest gaps remains a bold hypothesis that needs to be tested further.
Joint effect of topography and forest gap dynamics on tropical diversity
We identified two orthogonal factors promoting diversity and coexistence among closely-related species with different characteristics and affecting different diversity levels. Topography, spatio-temporally stable, mainly affects trees among species and promotes coexistence with niche partitioning (Chapter 3). Forest gap dynamics, varying in space and time, mainly affect trees within species and promote species coexistence with emergent neutrality (Chapter 4). Anticipating their joint effect on species coexistence and tropical diversity is therefore not straightforward. Simulation approaches, discussed in the next section, can help test hypotheses on spatial and temporal scales that experimental studies on tropical forests cannot achieve.
From a static temporal point of view, the topography and dynamics of forest gaps both favour the coexistence of species with divergent processes on orthogonal niches. Consequently, genotypic adaptations within species decrease the risk of a stochastic local extinction while species adaptations stabilize local coexistence. But the limits arise with temporal variation, which questions the origin of the system, the stability of genotypic adaptations to the dynamics of forest gaps, the stability of species via divergence along topography despite gene flow, and the strength of niche differences and emerging neutrality to avoid competitive exclusion. Indeed, we showed overlapping niches along the topography despite niche differentiation and a low level of differentiation among species along successional niches despite genotypic adaptations (Chapters 3 and 4). Consequently, we do not know to what extent the neutrality of the distribution margin of the species, for example with the convergence of functional traits (Chapters 2), and along the successional niches, with the genotypic adaptations of the species, will make it possible to overcome competitive exclusion (Turcotte and Levine 2016). Similarly, the adaptive genetic component of topographic position in which individuals grow is not negligible (chapter 3) and may suggest evolution towards and increased differentiation of species niches (Hoffmann and Merilä 1999), but the interference of reproduction with gene flow could disrupt the divergence of species with topographic niches and hinder the coexistence of sympatric species (Hochkirch et al. 2007). This highlights the need to further document the processes responsible for partial reproductive isolation in our models. In addition, we do not know the stability of genotypic adaptations to the dynamics of forest gaps, and one could imagine the evolution of species, or even a speciation with barriers to reproduction among genotypes, towards a specialized niche of succession (Lenormand 2012). Finally, the ideal would be to be able to explain the conditions of emergence of this subtle eco-evolutionary dynamics. We need to explain how a genotype can rise to increased frequency in the population in response to the spatio-temporal dynamics of forest gap dynamics, such as, for example, the characterisation of the conditions for the stable existence of a flammability genotype in a forest undergoing fire dynamics (Schwilk and Kerr 2002). And we need to explain how species diverged along the topography with partial reproductive isolation despite gene flow, either with reinforcement after secondary contact (Haavie et al. 2004) or with sympatric speciation (Savolainen et al. 2006). The preceding points underline the importance of the temporal dynamics of our system, but we have limited our analyses and discussions to the sympatric and microgeographic scale, and we may need to explore larger spatial domains (Estes et al. 2018), such as the regional scale, especially considering the large spatial dynamics of forest gaps (Chambers et al. 2013).
Limits and future directions to address eco-evolutionary dynamics of tropical tree coexistence
Stochasticity and neutrality are of paramount importance in merging ecology and evolution in an interdisciplinary approach
I argue that stochasticity and neutrality are neglected, especially by non-specialists and scientists from other disciplines, in understanding the coexistence of species in ecology and evolution, especially with regard to the neutral theory of molecular evolution (Kimura 1968) and the unified neutral theory of biodiversity (Hubbell 2001). From an evolutionary point of view, considering adaptations without drift and gene flow prevents the full exploration of species coexistence, forcing a deterministic view of evolution towards an easy establishment of species coexistence. Andrews et al. (2012) revealed the many misconceptions about genetic drift among biology undergraduates. Similarly, from an ecological point of view, the partitioning of niches without considering the emergence of neutral processes prevents the complete exploration of the coexistence of species, forcing a deterministic vision of ecology towards the predominance of species niches. To my knowledge, no study has addressed the misconceptions about the unified neutral theory of biodiversity among undergraduate students, but I have little doubts on their existence (B. Hérault pers. com.). Finally, at the crossroads of ecology and evolution, considering niche differentiation and emerging neutrality without reproductive isolation also prevents the full exploration of species coexistence (Weber and Strauss 2016). I do not claim that specialists in one field neglect neutral processes in their field, but that specialists in one discipline often neglect neutral processes in the other field, despite their common origin, preventing the good development of interdisciplinarity in ecology and evolution. Overall, I think the lack of appreciation or understanding of stochastic and neutral processes in ecology and/or evolution is an obstacle to the development of a good interdisciplinary approach, but may result from a deterministic teaching of both disciplines when simplifying the discourse. I thus plead for a good appreciation of the complexity of ecology and evolution in order to avoid neglecting the cryptic eco-evolutionary dynamics (Kinnison et al. 2015).
Limits of local-scale low-cost sequence capture to study the evolution of non-model species complexes in the wild
My PhD thesis was the opportunity to contribute to the development of low-cost sequence capture for the study of eco-evolutionary processes with the financial support of the innovative LOCOCAP project granted by INRA in 2019. Our goal was to develop affordable tools for genomic studies, i.e., produce a few mega base pairs of sequence data, in non-model species, such as tropical trees, a necessary step for the successful completion of my PhD project. My results have revealed new affordable avenues opened up by these techniques, including population genomics, environmental association analyses and genome-wide association studies, while my experience has pointed to some methodological problems that may be encountered and that will need to be taken into account in future gene capture experiments. The first limit is the existing preliminary genetic resources needed to design the target sequences. We benefited from diverse genetic resources for Symphonia [Olsson et al. (2017); Scotti et al., in prep; Tysklind et al., in prep; Torroba-Balmori et al., unpublished], including both genomes and transcriptomes, compared to the reduced availability of genomic resources in the more complex Eschweilera model [Vargas et al. (2019); M. Heuertz pers. com.]. The cross-validation of genetic resources for Symphonia could explain in part the better success of the gene capture experiment compared to the one of Eschweilera with the resulting high quality of targeted sequences (chapter 3). Indeed, the probes design of Symphonia was more complex and based on better-quality reference data than the one of Eschweilera. But apart from the importance of the preliminary bioinformatics steps, I believe that the wet laboratory steps were the most involved in the difference in success between the gene capture experiments of Symphonia and Eschweilera. The issue of unequal read abundance among sequenced libraries was a big impediment to Eschweilera analyses, resulting in a lot of missing data despite low levels of reads sequenced off-targets compared to Symphonia. I blame a bad balance of libraries in pools during the wet lab for this problem of the representativeness of the libraries. Low-cost capture was enabled by a reduction in reaction volumes coupled with increased library indexing, which may have amplified pipetting errors. Finally, post-capture bioinformatics revealed two other issues explaining discrepancies between the gene capture experiments of Symphonia and Eschweilera: sequencing quality and paralogs. Eschweilera clade Parvifolia species are diploid but some include a strong signature of a past genome duplication (Heuertz et al. 2020). The past genome duplication may explain the inconsistencies of the SNP call in Eschweilera when using the Symphonia pipeline. We therefore had to use a strict SNP call for the Eschweilera data in order to guarantee the reliability of the SNPs, while favouring missing data. Local sampling also limited the inference of population evolutionary history due to short scale sampling of widespread species without phylogeny and range-wide niche characterization. Local sampling has been associated with the sampling of a cryptic species in Symphonia and more generally with the lack of good sampling of “genetically pure” populations compared to “hybrid” populations, which has prevented a good exploration of adaptive introgression with the various current methodologies (Gompert and Buerkle 2009, Racimo et al. 2018, Burgarella et al. 2019, Pfeifer et al. 2019). Despite the enormous sampling effort of hundreds of mature trees in tropical forests, the limited number of individuals per species and the associated population structure decreased the statistical power of our analyses and limited the detection of markers in genome-wide association studies (GWAS; Korte and Farlow 2013) while preventing the convergence of Markov chains in the Bayesian inference of polygenic genomic signals (Zhou et al. 2013) (although X. Zhou pers. com. considered our results as still valid). In conclusion, future gene capture experiments should consider: (i) the spatial scale, population structure and number of individuals, (ii) the existing preliminary genetic resources and the particularities of the evolution of the model, such as past duplication of the genome, (iii) the wet lab protocol with emphasis on library balance in capture pools, and (iv) the pipeline used for SNP call in post-sequencing analysis.
Promising directions in evolutionary studies of forest tree coexistence
Chapters 3 and 4 have pointed to several evolutionary areas and concepts, which I consider to be under-explored and promising in future studies of the coexistence of forest trees. Microgeographic adaptations point to a small and local spatial scale for evolution (Richardson et al. 2014) where sympatric coexistence of forest trees occurs. The hypothesis that evolution and ecology play at very different spatial scales may have been an obstacle to understanding the role of microgeographic adaptations (Pelletier et al. 2009), but previous studies (Brousseau et al. 2015) and chapters 3 and 4 have revealed the importance of microgeographic adaptation in the coexistence of trees within and between species in tropical forests, highlighting its importance in future studies. Similarly, overlapping generations are not exploited in evolutionary studies of tropical trees with widely overlapping generations (chapter 4), despite their importance in evolutionary theories (Ellner and Hairston Jnr 1994). Chapter 4 provides, to our knowledge, the first evidence for the role of forest gap dynamics on adaptation within tropical tree species. But our results are limited to three species in one species complex, and they open a quantity of new research avenues, such as the bold hypothesis of niche-hiking species in forest gaps (Schwilk and Kerr 2002). Studies on the genomics of forest gap dynamics need the previously highlighted concepts and may redefine the importance of forest gap dynamics in eco-evolutionary dynamics of tropical forest ecosystems, as forest gap dynamics is already recognized in ecology as a driving force of sylvogenesis. Microgeographic adaptations and overlapping generations played both a role in adaptation to forest gap dynamics detected in Symphonia. They highlight the fine spatio-temporal dynamics of forest gap dynamics. I thus more generally think that a promising direction in evolutionary studies of forest tree coexistence is a better understanding of the spatial and temporal domains of modern ecology and evolution (Estes et al. 2018). My PhD focused on a smaller spatial scale than usual with perhaps a larger temporal scale than usual, at least in ecology. This focus on specific spatial and temporal study scales has led to the uncovering of under-studied processes. However, these processes will still need to be included into regional spatial and longer temporal study scales, so as to further establish and characterize their role in the coexistence of species within the Amazon basin.
Simulation approaches to address the joint effects of topography and forest gap dynamics on the conditions for tree species coexistence
Eco-evolutionary observational studies on the coexistence of species in tropical forests are limited in forest stand inventories due to a lack of repeatability (Poorter et al. 2016 , Schnitzer and Carson 2016) and a lack of time to evaluate long-term effects (Hérault and Piponiot 2018). Forest simulators have proved useful to investigate eco-evolutionary processes through virtual experiments (Schmitt et al. 2019). I conducted a preliminary study on the development of an individual-based and spatially-explicit eco-evolutionary simulator to address the joint effects of topography and forest gap dynamics on the conditions for species coexistence and emergence. I specifically wanted to address the following questions:
- What are the conditions for the stable coexistence of genotypes and species with topography and/or forest gap dynamics in the forest community?
- What are the conditions for the successful establishment of genotypic adaptations to forest gap dynamics in the forest community?
- What are the conditions for the reinforcement of species barriers or speciation of species connected through gene flow along topography?
I hope to develop these questions in a future contribution, but I could only explore the definition of a simplified eco-evolutionary simulator based on Schwilk and Kerr (2002) and Vincenzi and Piotti (2014), including individuals and their explicit spatial positions, which is still far from having the capacity to explore these specific questions. I built a one-dimensional simulator, for example an environmental gradient, and a two-dimensional simulator, for example a spatially explicit environmental matrix, with exactly the same processes. The environmental gradient (one-dimension) is defined by a length and the environmental matrix (two-dimensions) is defined by a grid of size , with an individual growing on each cell. The environmental gradient (one-dimension) varies from the environmental minimum value at an extreme to the maximum value at the other extreme, and the environmental matrix (two-dimensions) varies in a grid defined by the product of the vectors . Each phenotype of individual is defined as the sum of its genotypic breeding value and an environmental effect , both drawn from a genetic and an environmental normal distribution:
At the initialization step, i.e. the first generation, or individuals are randomly drawn from the previous distribution and recruited over the environmental space. At each time step, i.e., at each generation that does not overlap with others, seedlings are produced in each cell. Seedlings inherit from two parents in the dispersal neighbourhood . Genotypic breeding values of seedlings are drawn from normal distributions centered on parental means and of half the variance of genetic variation (Vincenzi and Piotti 2014), and are used to compute seedling phenotypic value :
Among the seedlings one is recruited either with a determinist viability,
e.g. keeping the seedling with the phenotypic value closer to the seedling environmental value,
or with a probabilistic viability, e.g. with a random draw with probability equal to the inverse of the distance between seedling phenotypic value and environmental value.
I implemented both simulators in a web interface using C++ and R with the Rcpp (Eddelbuettel and François 2011) and shiny (Chang et al. 2019) packages.
The code is available in Appendix 1 (Simulator S1 and Simulator S2) and the simulators are available online (one-dimension simulator: https://sylvainschmitt.shinyapps.io/simulator1D/, two-dimensions simulator: https://sylvainschmitt.shinyapps.io/simulator2D/).
As a result of the ongoing work, both simulators have significant limitations or limiting assumptions, or even serious shortcomings, which are discussed below.
But using many generations () and default parameters in the two-dimensional simulator,
I was able to produce two divergent “pure” populations with a hybrid population at their interface (Fig. 16).
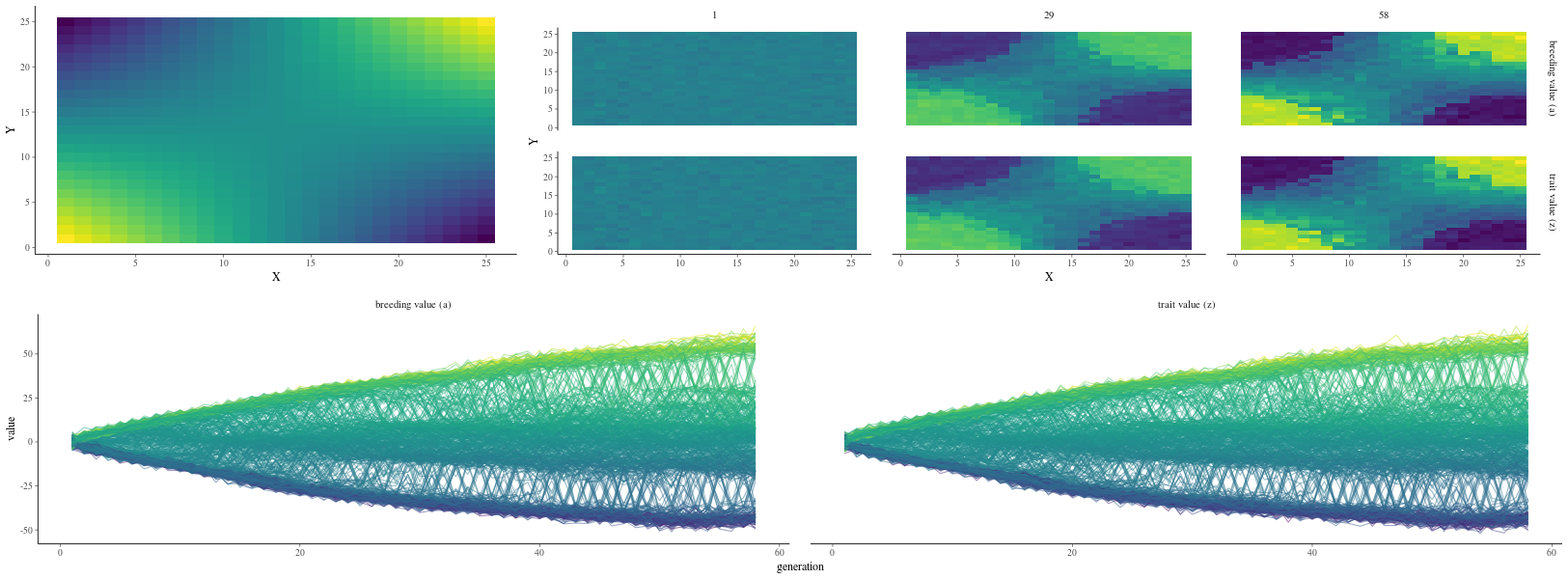
Figure 16: Results of the two-dimensional simulator with default settings over 58 generations. The upper left subplot shows the environmental matrix where the individuals develop, from -E in blue to E in yellow. The upper right subplot shows the breeding values a (upper row) and phenotypic values z (lower row) in the matrix for 3 different generations (the 1st in the left column, the 29th in the central column and the 58th in the right column), from -E in blue to E in yellow. The bottom subplot shows the breeding values a (left column) and phenotypic values z (right column) for each cell in the matrix (one line) with its associated environmental value, from -E in blue to E in yellow, over the 58 generations.
A serious shortcoming regarding the simulators is the dispersion. The shape of the dispersal kernel in one dimension is a line of length , which is correct, but it’s a square of size and not a discrete disk in two dimensions, which seems a strong bias. Moreover the seedling inherits from two parents in the dispersal neighbourhood , whereas the seedling should inherit from a mother tree at a distance from the seedling and from a father tree at a distance from the mother tree. Limiting assumptions include (i) the use of normal distributions that I wish to replace by lognormal distributions to better fit the models developed in chapters 3 and 4, and (ii) the use of non-overlapping generations that I wish to replace by a timeline defined by treefall events and overlapping generations defined by an explicit definition of individual mortality through background and treefall mortality. Future directions include the development of proposed solutions for shortcomings and limiting assumptions and the definition of environmental gradients. I am planning to develop a topography generator with spatial autocorrelation matching the one observed in Paracou, or that can vary with a few parameters. In case of failure in the development of a topography generator, I am planning to discretize and simplify observed topographic gradients in Paracou plots. Last but not least, I plan to model the dynamics of forest gaps as an extrinsic factor, with each cell growing in neighbourhood crowding at each time step, and with spatio-temporal stochastic events leading to treefall, i.e. the death of individuals in the area of the treefall and the definition of the local neighbourhood crowding at low values. In doing so, I wish to address the joint effect of the topography and dynamics of forest gaps on the conditions of coexistence and emergence of species, and to explore their joint effect on the hyperdiversity of tropical forests.
The primordial role of individuals within species in tropical forest diversity
Despite the dominant role of species in community ecology, the results of my PhD thesis have never ceased to underline the primordial role of individuals within species in the diversity of tropical forests. Chapter 2 revealed the importance of individuals in response to variations in topography, with an essentially plastic response of functional traits and weak genotypic adaptations introduced in chapter 3. Chapter 4 revealed the importance of individuals in response to forest gap dynamics, with strong genotypic adaptations, including an adaptive performance response with individual growth. In addition, the partitioning of the variance of individual growth potential, without genotypic effect, between inter- and intra-species variation revealed only a weak role of species in individual growth trajectories in the species complexes Symphonia and Eschweilera clade Parvifolia (7 to 8%, Fig. 27). Indeed, Clark et al. (2011) and Read et al. (2016) have shown that considering species without the individuals that make them up can blur community processes, highlighting the need to consider intraspecies variation in order to develop a more robust theory of community ecology. A first step suggested by Read et al. (2016) and applied in chapters 3 and 4, is the use of hierarchical models associated with variance partitioning (Nakagawa and Schielzeth 2013).
Nevertheless, the importance of individuals within species in ecological communities is not a new idea. Forty years ago, Aarssen (1983) already stressed the need to take into account individual variability within species to define both the fundamental niche and the competitive ability. Our results on topography (chapters 1 to 3) relate indirectly to the fundamental niche, besides being measured in the realized niche; whereas our results on forest gap dynamics (chapters 1 and 4) relate to competitive ability. Interestingly, Aarssen (1983) suggested that competition was an important force in natural selection, echoing the results found in chapter 4. Callaway et al. (2012) suggested that phenotypic plasticity promotes the coexistence of species with the adjustment of species to their communities. We found that plasticity is an individual response to neighbourhood crowding, but that adaptation processes also play an important role in the adaptation of species to their neighbours. Clark et al. (2010) suggested intraspecific variation to be structured and not random, as revealed by leaf functional traits variation with ontogeny, light access, and topography (chapter 2), and adaptive growth response to forest gap dynamics (chapter 4). In addition, Clark (2010) suggested that the individual variation within species allows species to differ in their responses to the environment, even though species may not differ on average. Conversely, Chapter 2 showed that the response of individuals within species to topography among species for most functional traits, despite some divergent responses such as for leaf chlorophyll content. We argued that responses of individuals to topography that are consistent across species could favor neutral processes among species along the topography when species overlap (Le Bagousse-Pinguet et al. 2014), despite mean trait differences among species (chapter 2). The hypothesis following our observations differs thus from Clark (2010)’s hypothesis, but I think they are complementary and not contradictory, such as niche and neutral theories (Gravel et al. 2006, Hérault 2007). Some traits may favour neutrality among species with different mean values but consistent response of individual variation within species to environmental gradients; while other traits may favour divergence among species with similar mean values but different responses of individual variation within species to environmental gradients. Intraspecific variability may help individuals within species to be “sufficiently different or sufficiently similar” among species (Scheffer and Van Nes 2006). Anyway, chapters 2 and 4 both support the idea that intraspecific trait variability promotes species coexistence by allowing species to pass through both the abiotic filter, with topography (chapter 2), and the biotic filter, with forest gap dynamics (chapter 4).
Consequently, the results of my PhD thesis, combined with previous literature, strongly argue in favour of a better consideration of individual variation within species in order to understand coexistence of species and, ultimately, community assembly. Just as Salguero-Gómez et al. (2018) calls for the merging of trait-based and demographics approaches among species, I believe that the merging of population genomics and demography among individuals within species holds promise for a better understanding of species coexistence. Clark (2010) already emphasized the similarity between population genomics and demography:
“Just as variation among individuals is required to maintain species by natural selection, providing a means for adaptive evolution in response to many factors in many dimensions, variation at the individual scale is also needed to explain why large numbers of intensely competing species coexist.”
I do not question the usefulness of the species concept in ecology, as long as we recognize its limitations, because the species concept has helped and is helping to build community ecology (Mcgill et al. 2006), and including individual variation within species is not simple (Albert et al. 2011) and can be an enormous amount of work. But I sincerely think that we should develop a theory of community ecology starting with individuals, because interactions with environments happen after all at the individual level (Violle et al. 2012).
References
Aarssen, L. W. 1983. Ecological Combining Ability and Competitive Combining Ability in Plants: Toward a General Evolutionary Theory of Coexistence in Systems of Competition. - The American Naturalist 122: 707–731.
Abbott, R. et al. 2013. Hybridization and speciation. - Journal of Evolutionary Biology 26: 229–246.
Albert, C. H. et al. 2011. When and how should intraspecific variability be considered in trait-based plant ecology? 13: 217–225.
Allié, E. et al. 2015. Pervasive local-scale tree-soil habitat association in a tropical forest community. - PLoS ONE 10: e0141488.
Amadon, D. 1966. The superspecies concept. - Systematic Zoology 15: 245–249.
Andrews, T. M. et al. 2012. Biology undergraduates’ misconceptions about genetic drift. - CBE Life Sciences Education 11: 258–259.
Baldeck, C. A. et al. 2013a. A taxonomic comparison of local habitat niches of tropical trees. - Oecologia 173: 1491–1498.
Baltzer, J. L. et al. 2005. Edaphic specialization in tropical trees: Physiological correlates and responses to reciprocal transplantation. - Ecology 86: 3063–3077.
Baraloto, C. et al. 2007. Seasonal water stress tolerance and habitat associations within four Neotropical tree genera. - Ecology 88: 478–489.
Baraloto, C. et al. 2012a. Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities. - Journal of Ecology 100: 690–701.
Barthe, S. et al. 2017. Tropical rainforests that persisted: inferences from the Quaternary demographic history of eight tree species in the Guiana shield. - Molecular Ecology 26: 1161–1174.
Bazzaz, F. A. and Carlson, R. W. 1982. Photosynthetic acclimation to variability in the light environment of early and late successional plants. - Oecologia 54: 313–316.
Brousseau, L. et al. 2015. Neutral and Adaptive Drivers of Microgeographic Genetic Divergence within Continuous Populations: The Case of the Neotropical Tree Eperua falcata (Aubl.) (FA Aravanopoulos, Ed.). - PLOS ONE 10: e0121394.
Bruelheide, H. et al. 2018. Global trait–environment relationships of plant communities. - Nature Ecology & Evolution: 1.
Burgarella, C. et al. 2019. Adaptive introgression: An untapped evolutionary mechanism for crop adaptation. - Frontiers in Plant Science 10: 1–17.
Callaway, R. M. et al. 2012. Phenotypic Plasticity and Interactions among Plants. - America 84: 1115–1128.
Cannon, C. H. and Lerdau, M. 2015. Variable mating behaviors and the maintenance of tropical biodiversity. - Frontiers in Genetics 6: 183.
Cannon, C. H. and Lerdau, M. T. 2019. Demography and destiny: The syngameon in hyperdiverse systems. - Proceedings of the National Academy of Sciences: 201902040.
Cannon, C. H. and Petit, R. J. 2019. The oak syngameon: more than the sum of its parts. - New Phytologist: nph.16091.
Caron, H. et al. 2019. Chloroplast DNA variation in a hyperdiverse tropical tree community. - Ecology and Evolution 9: ece3.5096.
Caujapé-Castells, J. et al. 2017. Island ontogenies, syngameons, and the origins and evolution of genetic diversity in the Canarian endemic flora. - Perspectives in Plant Ecology, Evolution and Systematics 27: 9–22.
Chambers, J. Q. et al. 2013. The steady-state mosaic of disturbance and succession across an old-growth central Amazon forest landscape. - Proceedings of the National Academy of Sciences of the United States of America 110: 3949–3954.
Chang, W. et al. 2019. shiny: Web Application Framework for R.
Chesson, P. 2000a. Mechanisms of maintenance of species diversity. - Annual review of Ecology and Systematics 31: 343–366.
Chevin, L. M. et al. 2010. Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory (JG Kingsolver, Ed.). - PLoS Biology 8: e1000357.
Clark, J. S. 2010. Individuals and the Variation Needed for High Species Diversity in Forest Trees. - Science 327: 1129–1132.
Clark, J. S. et al. 2010. High-dimensional coexistence based on individual variation: A synthesis of evidence. - Ecological Monographs 80: 569–608.
Clark, J. S. et al. 2011. Individual-scale variation, species-scale differences: Inference needed to understand diversity. - Ecology Letters 14: 1273–1287.
Dalling, J. W. and Hubbell, S. P. 2002. Seed size, growth rate and gap microsite conditions as determinants of recruitment success for pioneer species. - Journal of Ecology 90: 557–568.
Dick, C. W. and Heuertz, M. 2008. The complex biogeographic history of a widespread tropical tree species. - Evolution 62: 2760–2774.
Dick, C. W. et al. 2004. Molecular Systematic Analysis Reveals Cryptic Tertiary Diversification of a Widespread Tropical Rain Forest Tree. - The American Naturalist 162: 691–703.
Eddelbuettel, D. and François, R. 2011. Rcpp : Seamless R and C++ Integration. - Journal of Statistical Software in press.
Eiserhardt, W. L. et al. 2017. Plant phylogeny as a window on the evolution of hyperdiversity in the tropical rainforest biome. - New Phytologist 214: 1408–1422.
Ellner, S. and Hairston Jnr, N. G. 1994. Role of overlapping generations in maintaining genetic variation in a fluctuating environment. - American Naturalist 143: 403–417.
Engelbrecht, B. M. et al. 2007. Drought sensitivity shapes species distribution patterns in tropical forests. - Nature 447: 80–82.
Estes, L. et al. 2018. The spatial and temporal domains of modern ecology. - Nature Ecology and Evolution 2: 819–826.
Ewédjè, E. E. B. K. et al. 2020. Species delimitation in the African tree genus Lophira (Ochnaceae) reveals cryptic genetic variation. - Conservation Genetics 21: 501–514.
Ferry, B. et al. 2010a. Higher treefall rates on slopes and waterlogged soils result in lower stand biomass and productivity in a tropical rain forest. - Journal of Ecology 98: 106–116.
Fine, P. V. A. et al. 2004. Herbivores promote habitat specialization by trees in Amazonian forests. - Science (New York, N.Y.) 305: 663–5.
Gao, S. B. et al. 2018. Phenotypic plasticity vs. local adaptation in quantitative traits differences of Stipa grandis in semi-arid steppe, China. - Scientific Reports 8: 1–8.
Gentry, A. H. 1974. Flowering Phenology and Diversity in Tropical Bignoniaceae. - Biotropica 6: 64.
Ghalambor, C. K. et al. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. - Functional Ecology 21: 394–407.
Gompert, Z. and Buerkle, C. A. 2009. A powerful regression-based method for admixture mapping of isolation across the genome of hybrids. - Molecular Ecology 18: 1207–1224.
Gonzalez, M. A. et al. 2009. Identification of amazonian trees with DNA barcodes. - PLoS ONE 4: e7483.
Goulet, F. and Bellefleur, P. 1986. Leaf morphology plasticity in response to light environment in deciduous tree species and its implication on forest succession. - Canadian Journal of Forest Research 16: 1192–1195.
Gravel, D. et al. 2006. Reconciling niche and neutrality: The continuum hypothesis. - Ecology Letters 9: 399–409.
Gunatilleke, C. V. S. et al. 2006. Species–habitat associations in a {Sri} {Lankan} dipterocarp forest. - Journal of Tropical Ecology 22: 371.
Haavie, J. et al. 2004. Flycatcher song in allopatry and sympatry - Convergence, divergence and reinforcement. - Journal of Evolutionary Biology 17: 227–237.
Heuertz, M. et al. 2020. The hyperdominant tropical tree Eschweilera coriacea (Parvifolia clade, Lecythidaceae) shows higher genetic heterogeneity than sympatric Eschweilera species in French Guiana. - Plant Ecology and Evolution. 153: 67–81.
Hérault, B. 2007. Reconciling niche and neutrality through the Emergent Group approach. - Perspectives in Plant Ecology, Evolution and Systematics 9: 71–78.
Hérault, B. and Piponiot, C. 2018. Key drivers of ecosystem recovery after disturbance in a neotropical forest. - Forest Ecosystems 5: 2.
Hoban, S. et al. 2012. Computer simulations: Tools for population and evolutionary genetics. - Nature Reviews Genetics 13: 110–122.
Hochkirch, A. et al. 2007. Sympatry with the devil: Reproductive interference could hamper species coexistence. - Journal of Animal Ecology 76: 633–642.
Hoffmann, A. A. and Merilä, J. 1999. Heritable variation and evolution under favourable and unfavourable conditions. - Trends in Ecology & Evolution 14: 96–101.
Holliday, T. W. 2006. Neanderthals and modern humans: an example of a mammalian syngameon? - Vertebrate Paleobiology and Paleoanthropology: 281–297.
Huang, Y. Y. et al. 2015. Toward a phylogenetic-based generic classification of neotropical lecythidaceae—I. Status of Bertholletia, Corythophora, Eschweilera and Lecythis. - Phytotaxa 203: 085–121.
Hubbell, S. P. 2001. The unified neutral theory of biodervisity.
Itoh, A. et al. 2003. Importance of topography and soil texture in the spatial distribution of two sympatric dipterocarp trees in a Bornean rainforest. - Ecological Research 18: 307–320.
Kammesheidt, L. 2000. Some autecological characteristics of early to late successional tree species in Venezuela. - Acta Oecologica 21: 37–48.
Kay, K. M. 2006. REPRODUCTIVE ISOLATION BETWEEN TWO CLOSELY RELATED HUMMINGBIRD POLLINATED NEOTROPICAL GINGERS. - Evolution 60: 538–552.
Kimura, M. 1968. The neutral theory and molecular evolution. - In: My thoughts on biological evolution. Springer, ppp. 119–138.
Kinnison, M. T. et al. 2015. Cryptic eco-evolutionary dynamics. - Annals of the New York Academy of Sciences 1360: 120–144.
Koch, G. W. et al. 2004. The limits to tree height. - Nature 428: 851–854.
Korte, A. and Farlow, A. 2013. The advantages and limitations of trait analysis with GWAS: A review. 9: 29.
Kraft, N. J. B. et al. 2008. Functional traits and niche-based tree community assembly in an Amazonian forest. - Science 322: 580–582.
Lan, G. et al. 2016. Species associations of congeneric species in a tropical seasonal rain forest of China. - Journal of Tropical Ecology 32: 201–212.
Le Bagousse-Pinguet, Y. et al. 2014. Species richness of limestone grasslands increases with trait overlap: Evidence from within- and between-species functional diversity partitioning (D Wardle, Ed.). - Journal of Ecology 102: 466–474.
Leigh, A. et al. 2011. Structural and hydraulic correlates of heterophylly in Ginkgo biloba. - New Phytologist 189: 459–470.
Lenormand, T. 2012. From local adaptation to speciation: Specialization and reinforcement. - International Journal of Ecology in press.
Levi, T. et al. 2019a. Tropical forests can maintain hyperdiversity because of enemies. - Proceedings of the National Academy of Sciences 116: 581–586.
Levi, T. et al. 2019b. Reply to Cannon and Lerdau: Maintenance of tropical forest tree diversity. - Proceedings of the National Academy of Sciences 116: 8106–8106.
Levin, D. A. et al. 1996. Hybridization and the extinction of rare plant species. - Conservation Biology 10: 10–16.
Mcgill, B. et al. 2006. Rebuilding community ecology from functional traits. - Trends in Ecology & Evolution 21: 178–185.
Metz, M. R. 2012. Does habitat specialization by seedlings contribute to the high diversity of a lowland rain forest? - Journal of Ecology 100: 969–979.
Molino, J.-F. and Sabatier, D. 2001. Tree Diversity in Tropical Rain Forests: A Validation of the Intermediate Disturbance Hypothesis. - Science 294: 1702–1704.
Mutke, J. et al. 2014. Diversity patterns of selected Andean plant groups correspond to topography and habitat dynamics, not orogeny. - Frontiers in Genetics in press.
Nakagawa, S. and Schielzeth, H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. - Methods in Ecology and Evolution 4: 133–142.
Oldham, A. R. et al. 2010. The hydrostatic gradient, not light availability, drives height-related variation in Sequoia sempervirens (Cupressaceae) leaf anatomy. - American Journal of Botany 97: 1087–1097.
Olsson, S. et al. 2017. Development of genomic tools in a widespread tropical tree, Symphonia globulifera L.f.: a new low-coverage draft genome, SNP and SSR markers. - Molecular Ecology Resources 17: 614–630.
Paun, O. et al. 2016. Processes Driving the Adaptive Radiation of a Tropical Tree (Diospyros, Ebenaceae) in New Caledonia, a Biodiversity Hotspot. - Systematic Biology 65: 212–227.
Pelletier, F. et al. 2009. Eco-evolutionary dynamics. - Philosophical Transactions of the Royal Society B: Biological Sciences 364: 1483–1489.
Pérez-Harguindeguy, N. et al. 2013. New Handbook for standardized measurment of plant functional traits worldwide. - Australian Journal of Botany 61: 167–234.
Pfeifer, B. et al. 2019. Genome Scans for Selection and Introgression based on k-nearest Neighbor Techniques. - bioRxiv: 752758.
Pickrell, J. K. and Pritchard, J. K. 2012. Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data. - PLoS Genetics in press.
Pinheiro, F. et al. 2018. Plant Species Complexes as Models to Understand Speciation and Evolution: A Review of South American Studies. - Critical Reviews in Plant Sciences 37: 54–80.
Poorter, L. et al. 2016. Biomass resilience of Neotropical secondary forests. - Nature 530: 211–214.
Racimo, F. et al. 2018. Detecting Polygenic Adaptation in Admixture Graphs. - Genetics 208: 1565–1584.
Read, Q. D. et al. 2016. Accounting for the nested nature of genetic variation across levels of organization improves our understanding of biodiversity and community ecology. - Oikos 125: 895–904.
Richardson, J. L. et al. 2014. Microgeographic adaptation and the spatial scale of evolution. - Trends in Ecology and Evolution 29: 165–176.
Rieseberg, L. H. et al. 2003. Major ecological transitions in wild sunflowers facilitated by hybridization. - Science 301: 1211–1216.
Rundell, R. J. and Price, T. D. 2009. Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. - Trends in Ecology & Evolution 24: 394–399.
Sabatier, D. et al. 1997. The influence of soil cover organization on the floristic and structural heterogeneity of a Guianan rain forest. - Plant Ecology 131: 81–108.
Salguero-Gómez, R. et al. 2018. Delivering the promises of trait-based approaches to the needs of demographic approaches, and vice versa. - Functional Ecology 32: 1424–1435.
Savolainen, V. et al. 2006. Sympatric speciation in palms on an oceanic island. - Nature 441: 210–213.
Scheffer, M. and Van Nes, E. H. 2006. Self-organized similarity, the evolutionary emergence of groups of similar species. - Proceedings of the National Academy of Sciences of the United States of America 103: 6230–6235.
Schmitt, S. et al. 2019. Functional diversity improves tropical forest resilience: insights from a long-term virtual experiment. - Journal of Ecology: 1365–2745.13320.
Schnitzer, S. A. and Carson, W. P. 2016. Would Ecology Fail the Repeatability Test? 66: 98–99.
Schwilk, D. W. and Kerr, B. 2002. Genetic niche-hiking: An alternative explanation for the evolution of flammability. - Oikos 99: 431–442.
Seehausen, O. 2006. African cichlid fish: A model system in adaptive radiation research. - Proceedings of the Royal Society B: Biological Sciences 273: 1987–1998.
Steege, H. ter et al. 2013. Hyperdominance in the Amazonian Tree Flora. - Science 342: 1243092–1243092.
Taylor, E. B. et al. 2006. Speciation in reverse: Morphological and genetic evidence of the collapse of a three-spined stickleback (Gasterosteus aculeatus) species pair. - Molecular Ecology 15: 343–355.
Tigano, A. and Friesen, V. L. 2016. Genomics of local adaptation with gene flow. - Molecular Ecology 25: 2144–2164.
Tobias, J. A. et al. 2014. Species coexistence and the dynamics of phenotypic evolution in adaptive radiation. - Nature 506: 359–363.
Torroba-Balmori, P. et al. 2017. Altitudinal gradients, biogeographic history and microhabitat adaptation affect fine-scale spatial genetic structure in African and Neotropical populations of an ancient tropical tree species. - PLOS ONE 12: e0182515.
Turcotte, M. M. and Levine, J. M. 2016. Phenotypic Plasticity and Species Coexistence. - Trends in Ecology & Evolution 31: 803–813.
Umaña, M. N. and Swenson, N. G. 2019. Intraspecific variation in traits and tree growth along an elevational gradient in a subtropical forest. - Oecologia 191: 153–164.
Vargas, O. M. et al. 2019. Target sequence capture in the Brazil nut family (Lecythidaceae): Marker selection and in silico capture from genome skimming data. - Molecular Phylogenetics and Evolution 135: 98–104.
Vincenzi, S. and Piotti, A. 2014. Evolution of serotiny in maritime pine (Pinus pinaster) in the light of increasing frequency of fires. - Plant Ecology 215: 689–701.
Violle, C. et al. 2007. Let the concept of trait be functional! - Oikos 116: 882–892.
Violle, C. et al. 2012. The return of the variance: Intraspecific variability in community ecology. - Trends in Ecology and Evolution 27: 244–252.
Weber, M. G. and Strauss, S. Y. 2016. Coexistence in Close Relatives: Beyond Competition and Reproductive Isolation in Sister Taxa. - Annual Review of Ecology, Evolution, and Systematics 47: 359–381.
Yamasaki, N. et al. 2013. Coexistence of two congeneric tree species of Lauraceae in a secondary warm-temperate forest on Miyajima Island, south-western Japan. - Plant Species Biology 28: 41–50.
Zangerl, A. R. and Bazzaz, F. A. 1983. Plasticity and genotypic variation in photosynthetic behaviour of an early and a late successional species of Polygonum. - Oecologia 57: 270–273.
Zhou, X. et al. 2013. Polygenic Modeling with Bayesian Sparse Linear Mixed Models (PM Visscher, Ed.). - PLoS Genetics 9: e1003264.