Introduction
Biodiversity in tropical forests from the Amazon Basin
Tropical forests’ outstanding biodiversity
The maintenance of biodiversity is a long-standing issue for both ecology (Hutchinson 1941) and evolution (Darwin 1909). Biodiversity is characterized by nested levels, from genes, through individuals and species, to ecosystems. Ecosystems hold the biological community of interacting organisms. Earth presents a large number of terrestrial and marine ecosystems. Among them, the outstanding biodiversity of tropical rainforests has always fascinated biologists (Connell 1978). Tropical forests shelter the highest species diversity worldwide (Gaston 2000), a fact that remains partly unexplained and the origin of which is subject to debate (Wright 2002).
Threats that affect tropical forests
Tropical forest disturbances are increasing under the influence of human activities (Davidson et al. 2012, Lewis et al. 2015). Human activities induce direct disturbances on tropical forests, such as forest logging and fires (Pearson et al. 2017), or indirect disturbances, such as global change affecting regional climates (Lewis et al. 2004). For instance, climate change is predicted to increase the frequency of drought events (Davidson et al. 2012) and of convective storms (Negrón-Juárez et al. 2018), among other things. Increasing disturbance threatens many species around the world and is leading to a global and tropical biodiversity crisis (Cardinale et al. 2012). However, biodiversity itself helps tropical forests to cope with disturbances by increasing their resilience (Schmitt et al. 2019). Consequently, there is an increased and urgent need to understand and predict biodiversity dynamics in response to global change.
Regional and local species richness in the Amazon Basin
Among tropical forests, the Amazon Basin is the world’s most diverse terrestrial ecosystem. Neotropical rainforests harbour around one hundred thousand species of seed plants accounting for ca. 37% of all seed plants worldwide (Gentry 1982, Antonelli and Sanmartín 2011, Eiserhardt et al. 2017). Lowland Amazonia alone is estimated to shelter around sixteen thousand tree species (Steege et al. 2013). Even at the hectare-scale, tropical forests shelter up to several hundred tree species (Gentry 1988). Among tree species, a few defined as oligarchies are abundant from local to regional scale (Pitman et al. 2001). Similarly at the regional scale, 1.4% of the estimated total of Amazonian tree species represent more than half of the total number of observed stems above ten centimeter diameter at breast height and are thus called hyperdominant (Steege et al. 2013).
Local coexistence of species: ecological considerations
Niche and neutral theory
At the species level, different ecological theories have been developed to explain the coexistence of species and thus the persistence of biodiversity. Niche theory explains species local coexistence based on ecological niche differences limiting competitive exclusion (Weiher and Keddy 1995, Lortie et al. 2004a). The heterogeneity of resources distribution in space and time defines fine-scale habitat where species can coexist. For instance, the topography spatially drives water and nutrient distribution in tropical forest (John et al. 2007). Therefore, topography has a pervasive effect in differentiating habitat preference among species (Gunatilleke et al. 2006, Kraft et al. 2008, Allié et al. 2015). In particular, soil nutrients, also influenced by topography through hydromorphy, directly influence the spatial distribution of forest tree species (John et al. 2007, Jucker et al. 2018). Additionally, forest gap dynamics is a strong driver of above- and below-ground competition for resources in space and time (Hubbell et al. 1999, Breugel et al. 2012). Access to light has been recognized as a driver of closely-related species coexistence through habitat differentiation (Yamasaki et al. 2013). Conversely, neutral theory explains the local coexistence of functionally equivalent species through stochastic life, death, reproduction, and dispersal dynamics (Hubbell 2001). The emerging similarity theory reconciles niche and neutral theories by suggesting coexistence of distinct groups of species that are functionally similar within groups (Scheffer and Van Nes 2006, Hérault 2007). Functionally similar species must have similar fitness as an additional condition for stable coexistence (Chesson 2000a, Tobias et al. 2014, Turcotte and Levine 2016). In addition, fitness averaging among species can generate patterns congruent with the two theories, and is thus underlining the role of intraspecific variability in ecology (Chave 2004). But, numerous other theories exist to explain species coexistence (Wright 2002), in particular competition and forest succession among tropical tree species.
Neighbours and competition
Theories have also explored biotic interactions to explain the coexistence of species. The Janzen-Connell hypothesis explains species coexistence and the maintenance of tropical diversity with the interactions of two effects: (i) dispersal from parents, with more likely dispersal at short than long distance, and (ii) distance and density-dependent survival of offspring due to competition, pathogens, and herbivores, with more likely survival at long than short distance from parents (Clark and Clark 1984, Hyatt et al. 2003). Consequently, the inverted probability distribution with distance between the two effects results in a higher recruitment probability at intermediate distances according to the hypothesis. Other authors suggested theories focused on density-dependence. A model of symmetric density-dependent survival reproduced similar distributions of species abundances than abundance distributions found with neutral dispersal limitation models, advocating for the existence of one mechanism or the combination of density-dependent survival and neutral dispersal limitations in natural populations (Volkov et al. 2005). Moreover, asymmetric density-dependence of survival with different levels of competition with conspecific and heterospecific predicted well the rarity and abundance of species in tropical tree communities (Comita et al. 2010).
Treefall and succession
Beyond density-dependence, theories have explored the peculiar and paramount role of treefalls and succession in natural tropical forests. Aboveground competition for light due to forest gap dynamics is often greater than the effect of belowground competition for water and nutrient dynamics, even in early successional stages (Breugel et al. 2012). The intermediate disturbance hypothesis explains diversity with disturbance, positing a maximal diversity at intermediate regime of disturbances (Molino and Sabatier 2001). The intermediate disturbance hypothesis advocates thus for a primordial role of forest gap dynamics in natural tropical forests (Canham et al. 1990). After a treefall, pioneer species grow first in light-gaps, whereas late-successional species grow later under closed-canopy (Craven et al. 2015), defining successional niches among species (Herault et al. 2010). Abiotic factors also play a role in the distribution of treefalls and forest gap dynamics (Ferry et al. 2010a, Goulamoussène et al. 2017). Consequently, forest gap dynamics stress the importance to consider temporal as well as spatial dynamics to understand species coexistence (Soininen 2010).
Intraspecific variability
Despite the role of intraspecific variability in the forest community (Messier et al. 2010b, Siefert et al. 2015a), previous ecological theories often ignore individual diversity within species’ local populations (Chave 2004). However, several authors suggested the hypothesis or have shown evidence that intraspecific genetic or phenotypic variability promotes species coexistence (Lichstein et al. 2007). Following observations of community trait shifts due to intraspecific variability, Jung et al. (2010a) concluded that intraspecific trait variability promotes species coexistence, by facilitating species to pass through environmental filtering. Studying limestone grassland functional diversity, Le Bagousse-Pinguet et al. (2014) were able to relate increasing interspecific trait overlap through increased intraspecific variability to a greater species coexistence. Clark (2010) suggested that intraspecific variability allows species to differ in their distribution of responses to the environment and thus to pass environmental filtering, which would have occurred on species’ mean phenotype. Clark (2010) found this hypothesis to be consistent with theories that predict a coexistence of more species with competition being stronger within than between species. Similarly, Chesson (2000b) suggested that intraspecific variability promotes species coexistence through stabilizing mechanisms “if negative intraspecific interactions tend to be greater than interspecific interactions”. Some authors suggested that the intraspecific level should hold evidence of mechanisms promoting species coexistence and thus biodiversity. Laughlin and Laughlin (2013) suggested testing the limiting similarity hypothesis in the trait space incorporating intraspecific variability. And, Laughlin et al. (2012) suggested that intraspecific variability could answer paradoxes in theories of species coexistence.
Intraspecific variability might play a different role depending on the studied organism. Specifically, long-lived species, such as tropical trees, have high ontogenetic variation and phenotypic plasticity that might be a result from a high intraspecific variability to face environmental hazards before they reach sexual maturity (Sultan 1987, Borges 2009). Callaway et al. (2012) suggested that intraspecific variability, through phenotypic plasticity in response to neighbours, might promote community diversity and species coexistence, letting species adjust the composition of their populations. Aarssen (1983) suggested the “competitive combining ability” hypothesis which hypothesizes species coexistence to be based on the ability of each species to respond to spatial and variable selection imposed by neighbouring species. Following his hypothesis, the species’ ability to respond to selection promotes biodiversity through species coexistence and stabilizes species composition among communities (Vellend 2006).
Demography and fundamental trade-offs
Ultimately, the introduced theories try to predict species demography to explain species coexistence. Tree demography rests on individual performance in recruitment, growth, survival, and reproduction (Baraloto et al. 2005, Poorter and Bongers 2006, Violle et al. 2007, Román-Dañobeytia et al. 2012). The ability of trees to grow, survive, and reproduce can be measured by growth rates (Baraloto et al. 2005, Herault et al. 2010, Osazuwa-Peters et al. 2017), mortality rates (Aubry-Kientz et al. 2015a, Osazuwa-Peters et al. 2017)), and monitoring of cohorts and allele frequencies (Unger et al. 2016), respectively. Fundamental trade-off exists between performance components. For instance, tropical trees have been shown to present a trade-off between growth and survival (Buchman et al. 1983, Wright et al. 2010). In particular, a trade-off was identified between survival in shade and growth in gaps (Baraloto et al. 2005, Herault et al. 2010). Globally, some authors suggested the existence of a fast-slow continuum decoupled with a reproductive strategy to represent all plant demographic strategies (Salguero-Gómez 2017).
Functional traits: from fundamental trade-offs to ecological niches
To bridge the gap between ecological niches and demography, functional traits reflect fundamental trade-offs determining the species’ ecological niches along environmental gradients (Wright and Westoby 2002) and shape the structure (Paine et al. 2011) and dynamics (Hérault and Piponiot 2018) of species in conjunction with their environment (Kraft et al. 2008). Functional traits have been specifically defined as traits which impact fitness indirectly through their effect on performance (Violle et al. 2007). Functional traits can be numerous and classified into traits related to biochemistry, physiology, anatomy, morphology, and phenology.
Several studies have related tree performance to functional traits. Hérault et al. (2011) related tree growth to stem economics (sensu Chave et al. 2009) and adult stature. Aubry-Kientz et al. (2013) related tree mortality to wood density, maximum height, laminar toughness, and stem and branch orientation, highlighting the trade-off between fast- and slow-growing species (Reich 2014a). Philipson et al. (2014) found that wood density was shaping the growth-mortality trade-off. On the opposite, Poorter and Bongers (2006) found that leaf traits underlie the growth-mortality trade-off, with short-lived physiologically active leaves resulting in higher growth capacity but lower survival chance. Finally, less is known about functional drivers of reproduction performance with tropical trees, but Niklas and Enquist (2003) were able to estimate the annual reproductive biomass thanks to the two-thirds power of the aboveground biomass of standing trees.
In addition, functional strategies related to fundamental demographic trade-offs have been identified among tropical tree species, better bridging demographic and trait-based approaches (Salguero-Gómez et al. 2018). Functional traits covary among species (Díaz et al. 2016) and communities (Bruelheide et al. 2018) along distinct economics spectra of leaf (Wright et al. 2004, Osnas et al. 2013, but see Lloyd et al. 2013) and wood (Chave et al. 2009). The leaf economics spectrum opposes acquisitive ecological strategies, with high photosynthetic carbon assimilation, to conservative ones with high investment in leaf defence and durability (Wright et al. 2004). The wood economics spectrum opposes fast growing to slow growing species (Chave et al. 2009). Nevertheless, some authors advocate for a unique plant economics spectrum (Reich 2014a).
Adaptive evolution: from individual genomes to tree species
Bridging ecology and evolution: ecological genomics
Traditionally, theories and studies in community ecology focused on species, ignoring genotypic and phenotypic variation within species (Mcgill et al. (2006); but see the literature in the intraspecific variability paragraph). Consequently, ecological theories and related studies often ignore evolutionary forces driving the past and future of biodiversity. Conversely, geneticists are often focused on the function of genes outside of their natural environment. But “nothing in biology makes sense except in the light of evolution” (Dobzhansky 1973). The assumption that evolution and ecology play at very different spatial- and time-scales might have been an impediment to understand the role of eco-evolutionary processes in species coexistence (Pelletier et al. 2009). However, eco-evolutionary processes have been documented for a while (Tutt 1896) and can play an important role in the dynamics of both species and communities (Bailey et al. 2009). To bridge the gap between ecology and evolution, ecological genomics specifically explores the genetic mechanisms that underlie the responses of organisms to their natural environments (Savolainen et al. 2013, Holliday et al. 2017).
The genomic basis of adaptation
Genetic variability is the genetic differences we find within and among genomes of individuals within and among populations. Thus genetic variability is ultimately formed of polymorphisms. A locus can contain one or several polymorphisms resulting in several alleles of the given locus. Genetic variability always arises from new mutations inside the genome of an individual (e.g. Plomion et al. 2018). If mutations directly promote individual fitness through its phenotype in its local environment, they may become a source of adaptation. They can, thus, also contribute beneficial alleles to the pool of standing genetic variation into the population, allowing eventually for further adaptation of other individuals from the population (Barret and Schluter 2008). Finally, both new mutations and standing genetic variation can be transferred and be beneficial in other populations through adaptive introgression (Tigano and Friesen 2016). The establishment of beneficial new mutations and fixation of standing genetic variation will depend on genetic drift, and the strength of selection over gene flow, where gene flow can be disruptive but bear adaptive introgressions (Tigano and Friesen 2016).
A first step in understanding the link between phenotypic and genetic variation is to estimate the genetic component of phenotypic variation. Genotypic and population components of phenotypic variation can be assessed through the use of a mixed model, known as the animal model (Wilson et al. 2010). Next, a simple way to detect the genomic basis of adaptation is to map variants with an adaptive phenotype, using genome wide association studies (GWAS; Korte and Farlow 2013). The major issue is to correctly deal with population structure in natural populations to avoid false positive discovery, using for instance mixed models (Zhou and Stephens 2012). But phenotypes are not always available, and we can try to detect genomic adaptations based on the genetic signatures left by selection. Different models of selection have been developed and imply varying methodology to detect signatures of selection (Flood and Hancock 2017). The hard sweep model describes the fast selection of a new variant which rapidly increases to high frequency, ‘sweeping’ previous variation in the region due to hitchhiking of neutral variation (Smith and Haigh 1974). Consequently, the simplicity of this model allows the detection of the signature of selection thanks to the sweep based on linkage disequilibrium (Sabeti et al. 2002), allele frequency change (Fay and Wu 2000), and population differentiation (Gaggiotti and Foll 2010). Nevertheless, more complex scenarii can exist in nature. Soft sweep models predict multiple haplotypes with adaptive variants (Messer and Petrov 2013), jeopardizing traditional hard sweep detection. Indeed, many phenotypes investigated by evolutionists are expected to be polygenic, e.g. human height (Zeng et al. 2018). Finally, adaptive introgression represents the transfer between divergent lineages of adaptive variants thanks to hybridization. Adaptive introgression might be important among plants with hybrids occurring in 16% of genera (Whitney et al. 2010). All genetic adaptations originate within an individual’s genome but spread among individuals and ultimately species.
Individual microgeographic adaptations
Individual adaptation in trees has traditionally been studied among populations thanks to common gardens representing provenances sampled on wide ecological and spatial gradients (e.g. Dewoody et al. 2015). Individual adaptations among populations is called local adaptation, based on the fact that local populations tend to have a higher mean fitness in their native environment than in other environments (Savolainen et al. 2013, Lascoux et al. 2016). But fine spatio-temporal scale environmental variations can happen locally and lead to individual adaptations too. Evidence exists for eco-evolutionary processes at microgeographic scale, e.g. within the dispersal neighbourhood of the organism (Richardson et al. 2014), and under gene-flow (Savolainen et al. 2007, Tigano and Friesen 2016). Microgeographic adaptation participates in the local drivers of intraspecific variability with phenotypic plasticity (Benito Garzón et al. 2019). Topography and abiotic habitat have been evidenced to promote intraspecies divergences and microgeographic adaptations in Neotropical tree species (Brousseau et al. 2013, 2015). Other studies revealed microgeographic change in genetic structure and diversity of Neotropical trees without identifying adaptations (Jones and Hubbell 2006, Audigeos et al. 2013), including effect of topography (Torroba-Balmori et al. 2017), logging (Degen et al. 2006, Leclerc et al. 2015) and forest gaps (Scotti et al. 2015). Few studies further identified traits underlying genetic adaptations to local factors, like serotiny adaptations to fire in Meditarranean pine (Budde et al. 2014). Beyond the microgeographic adaptations of individuals within populations, local adaptation translates into adaptations of individuals between populations. But a strong disruptive selection between locally adapted populations to different habitats may be deleterious, revealing a potential transition from local adaptation to ecological speciation (Lascoux et al. 2016).
Species adaptive radiations
Savolainen et al. (2006) were able to evidence an event of sympatric ecological speciation with species sympatry, sister relationships, reproductive isolation, and highly unlikely earlier allopatric phase. But the latter might be hard to evidence in continental species. Closely-related species growing in sympatry in differentiated ecological niches form an adaptive radiation, such as Darwin’s finches in the Galapagos (Seehausen 2004, Grant and Grant 2019). Consequently, evolutionary history behind adaptive radiations falls within a continuum from sympatric ecological speciation to secondary contacts of species ecologically specialised in allopatry or parapatry (Rundell and Price 2009). Few studies evidenced adaptive radiation driven by topography or other abiotic habitats for tropical trees (Pillon et al. 2014, Paun et al. 2016).
Species complexes can result from adaptive radiations and species segregation along environmental gradients. Species complexes may combine and reshuffle genetic features among species in hybrid swarms (Seehausen 2004), exploring the whole niche breadth and multiplying the number of potential ecological niches, reducing competition among closely-related species, and helping species gain reproductive isolation (Runemark et al. 2019). Indeed, hybrid or derived species require some degree of reproductive isolation from their progenitors to avoid becoming an evolutionary melting pot. Nevertheless, simulations suggest that low hybridization success among closely-related species promotes coexistence of species in the community by allowing the survival of rare species through hybridisation (Cannon and Lerdau 2015). Species-specific adaptations may be maintained or even maximised under gene flow, especially with selective pressures varying in space and or time (Tigano and Friesen 2016). For instance, the European white oaks form one of the best known syngameons (Cannon and Petit 2019). European white oaks’ species lack private haplotypes indicating extensive hybridisation (Petit et al. 2002), but show unique ecological niches with regard to drought, cold, and tolerance to alkaline-soils (Leroy et al. 2019, Cannon and Petit 2019). The coexistence of the different species is partly due to the genes allowing their survival in different ecological niches (Leroy et al. 2019).
Eco-evolutionary peculiarities of trees
Forest trees are a major group of organisms due to their combined ecological, economic, and societal importance (Holliday et al. 2017). Trees have eco-evolutionary peculiarities (Petit and Hampe 2006), such as their great size, long life time, numerous seeds, large effective population sizes and overlapping generations (Brown et al. 2004, Heuertz et al. 2006). Tree seedlings undergo strong selection in early life stages (Kremer et al. 2012). Tree species often have outcrossing mating systems, maintain high levels of gene flow (Hamrick et al. 1992, Nybom 2004, Petit et al. 2004), and have low speciation rates (Petit and Hampe 2006). As a result, forest trees present a large degree of adaptation (Savolainen et al. 2013), and high levels of genetic variability. Woody species have been shown to harbour more genetic diversity within populations but less among populations than non woody species (Hamrick et al. 1992). Consequently, tropical trees are well suited to explore eco-evolutionary dynamics in closely-related species and their impact on Neotropical biodiversity.
Study context: the long-term study site of Paracou in a coastal forest of the Guiana Shield
The Guiana Shield
The Guiana Shield is covered by a Precambrian geologic formation (more than 1.9 Gyr old) of over 2 million square kilometers, in northern South America, from the Amazon river in the Brazilian state of Amapa in the South-East to the Orinoco river in Venezuela in the West (Hammond 2005). Soils developed from volcanic, plutonic, and metamorphic materials of the Paleoproterozoic (Delor et al. 2003), and are now heavily eroded, thick, and chemically poor (Ferry et al. 2003). Specifically in French Guiana, the elevation is relatively low (Bellevue mountains culminate at 851m) and topography is characterised by numerous small hills carving out seasonally-flooded bottomlands (Epron et al. 2006). Precise geomorphological characterisation and maps of French Guiana can be found in Guitet et al. (2013).
Guiana Shield geology and pedology compared with lowland western Amazon results in a major gradient in soil fertility directly affecting tree composition and function across Amazonia (Steege et al. 2006). Soil properties, including organic carbon, phosphorous, arbuscular mycorrhizal fungi, clay, and toxic elements, drive forest dynamics, through tree growth and mortality, in the phosphorus-depleted Guiana Shield (Soong et al. 2020). Poorer soils with slower nutrient cycling have lower tree growth but longer longevity compared to richer clay soils, which are better able to retain phosphorus and organic matter (Soong et al. 2020).
Ninety percent of the Guiana Shield is covered by tropical forests, whereas less than 2 million people inhabit the area, mainly on the coasts, which results in the highest level of forest cover per capita worldwide (Hammond 2005). Contrary to previous expectations, the Guiana Shield has been inhabited for a long time, with pre-Columbian occupations still influencing current forest structure and composition (Odonne et al. 2019). Although well conserved, the forests of the Guiana Shield are subject to numerous anthropogenic pressures including selective logging, gold mining, and land use change (Dezécache et al. 2017a). Selective logging affects 2 Mha of Amazonian forest per year but forests recover faster in the Guiana Shield than in western Amazonia (Piponiot et al. 2016). Gold mining primarily affects the forests of the Guiana Shield in South America with an exponential increase since the early 2000’s (Dezécache et al. 2017b).
The long-term study site of Paracou
The whole PhD study was conducted in the biologically- and scientifically-rich Paracou field station (latitude 5°18’N and longitude 52°53’W), in the coastal region of French Guiana. The site is characterized by an average annual rainfall of 3041 mm and a mean air temperature of 25.71 °C, with a marked seasonal variation (Aguilos et al. 2018). Topography is characterised by lowlands with heterogeneous micro-conditions due to numerous small hills generally not exceeding 45 m a.s.l. An old tropical forest with an exceptional richness (i.e. over 200 woody species per hectare) grows in this landscape with a dominance of Fabaceae, Chrysobalanaceae, Lecythidaceae, and Sapotaceae (Gourlet-Fleury et al. 2004).
A logging-experiment was initially launched with twelve 9-ha plots in 1984, further filled with three 6.25-ha, and one 25-ha undisturbed plots. Nine of the original plots were logged in 1986 with a range of disturbance intensities resulting in diverse biotic environments (details in Hérault and Piponiot 2018). Tree diameters at breast height (DBH) have been censused every 1-2 years since 1984. Trees have been mapped to the nearest meter and botanically identified, often to the species level. Weather and climate is continuously recorded thanks to an eddy flux tower (Bonal et al. 2008). Pedology and hydrology have been finely characterised for several plots (Ferry et al. 2010a). Finally, airborne LiDAR campaigns were conducted for several years at Paracou, which allowed the accurate characterization of forest topography and structure (Vincent et al. 2012).
This accumulation of high-quality environmental data in such a biologically-rich ecosystem led to numerous discoveries related to eco-evolutionary dynamics in Neotropical biodiversity. Among others, climate has a strong impact on forest dynamics, with, for instance, a drought resistance in species with high wood density and small stature favored by climate (Aubry-Kientz et al. 2015b). Topography effects on nutrient distribution and water availability drive forest dynamics (Ferry et al. 2010a) and result in pervasive habitat differentiation among species (Allié et al. 2015). Water availability (Wagner et al. 2011, 2012) and forest gaps (Herault et al. 2010) particularly drive tree growth. And a relation exists between tree growth and survival (Aubry-Kientz et al. 2013, 2015a). In addition to demography, co-variation of functional traits, their composition, and their relations to environment and demography have been explored (Baraloto et al. 2010a, Hérault et al. 2011). Finally, fine-scale genetic structures resulting from the identified ecological dynamics have also been documented. Gene dispersal inferences suggested limited gene and seed dispersal (gene dispersal from 150m to 1200m), possibly increasing local tree density (Degen et al. 2004, Hardy et al. 2005). Abiotic and biotic factors have been evidenced to structure genetic variation and diversity (Audigeos et al. 2013, Scotti et al. 2016), including topography (Brousseau et al. 2013), logging (Leclerc et al. 2015), and forest gaps (Scotti et al. 2015). But only few studies evidenced genetic adaptations (Brousseau et al. 2015). Therefore, the fine characterization of tropical forest diversity, fine abiotic and biotic spatio-temporal variations, and their eco-evolutionary relationships makes Paracou a perfect place to study eco-evolutionary dynamics in closely-related species of tropical trees.
Study models: Symphonia globulifera and Eschweilera clade Parvifolia two species complexes of locally abundant trees
Symphonia
Symphonia globulifera L. f. is a hypervariable pantropical tree species belonging to the Clusiaceae family. The Symphonia genus also includes a radiation of ca. 20 endemic sister-species in Madagascar (Perrier de la Bâthie 1951). S. globulifera is widespread from Guinea Bissau to Tanzania in the African Paleotropics and from Mexico to Brazil in the American Neotropics (Budde et al. 2013). Symphonia is an ancient genus with pollen identified in the Niger delta dated to 45Ma, that colonized the Americas from Africa ca. 18-16 Ma (Dick et al. 2004). S. globulifera is mainly outcrossing, even if selfing occurs, and disperses mostly over short distances (Degen et al. 2004, Hardy et al. 2005, Carneiro et al. 2009). S. globulifera is pollinated by hummingbirds, perching birds, and lepidoptera in the Neotropics and sunbirds in Africa (Torroba-Balmori et al. 2017). S. globulifera is dispersed by bats and tapirs in the Neotropics and small mammals in Africa (Torroba-Balmori et al. 2017). S. globulifera used many refugia during the Quaternary glaciations (Dick and Heuertz 2008, Budde et al. 2013). S. globulifera shows fine-scale genetic structure with topography throughout its distribution (Torroba-Balmori et al. 2017). Moreover, S. globulifera has possible ecotypic differentiation with topography and wetness, with two currently recognized morphotypes in French Guiana: S. globulifera sensu lato growing in seasonally-flooded bottomlands and S. sp1 growing in drier slopes and plateaus (Allié et al. 2015). In addition, the two morphotypes of French Guiana have different seasonal water stress tolerance (Baraloto et al. 2007), functional traits (Baraloto et al. 2010a, Fortunel et al. 2012) and growth potential (Hérault et al. 2011). Reciprocal transplantation experiments of Symphonia seedlings have shown that survival and growth performance of each morphotype is better in their home environment than in the opposite environment, showcasing how the two morphotypes are differently adapted to their respective environments (Tysklind et al., 2020). The two local morphotypes of S. globulifera might thus form a species complex in French Guiana (Gonzalez et al. 2009, Baraloto et al. 2012a). Genetic resources have been developed for S. globulifera with a published low-coverage genome sequenced in an individual from Africa (Cameroon; Olsson et al. 2017), an unpublished draft genome from an American individual (I. Scotti pers. com.), and an annotated transcriptome from an American individual, as well (N. Tysklind pers. com.).
Eschweilera
Eschweilera is a hyberabundant genus belonging to the Lecythidaceae family. Lecythis and Eschweilera genera are paraphyletic and consist of eight clades, including the Eschweilera clade Parvifolia (Huang et al. 2015, Mori et al. 2016). Abundant species of the Eschweilera clade Parvifolia in Paracou are E. coriacea (DC.) S.A.Mori, E. sagotiana Miers, and E. decolorans Sandwith (besides numerous others such as E. wachenheimii (Benoist) Sandwith and E. squamata S.A.Mori). Eschweilera coriacea is hyperdominant in all six Amazonian regions (Pitman et al. 2001, Steege et al. 2013) and showed high genetic heterogeneity compared to other sympatric closely-related species (Heuertz et al. 2020). Similarly to Symphonia morphotypes, E. sagotiana and E. coriacea exhibit niche differentiation with topography (Allié et al. 2015), different water stress tolerance (Baraloto et al. 2007), functional traits (Baraloto et al. 2010a, Fortunel et al. 2012) and growth potential (Hérault et al. 2011). Eschweilera clade Parvifolia is a species complex with low phylogenetic resolution and high plastid DNA sharing (Gonzalez et al. 2009, Baraloto et al. 2012a, Huang et al. 2015, Heuertz et al. 2020). Eschweilera species share more haplotypes among species than neutral expectations (Caron et al. 2019), suggesting the interspecific gene flow characteristic of syngameons. Eschweilera clade Parvifolia species are diploid but some include a strong signature of a past genome duplication (Heuertz et al. 2020). Genetic resources for Eschweilera calde Parvifolia include a Lecythidaceae bait set (Vargas et al. 2019) developed in silico and tested on previous genome skimming (Thomson et al. 2018).
Aims and general plan
The main objective of the PhD thesis was to explore the genotype-environment interactions in shaping individual phenotypic diversity within and among closely-related species belonging to species complexes of Neotropical trees. The study site for the thesis was the lowland rainforest in the research station of Paracou, French Guiana, where detailed inventory and tree growth data, as well as environmental characterization are available. I specifically wished to consider the intraspecific genomic variability as a continuum within structured populations of closely related species, and measure its role on individual tree performance through growth over time, while accounting for effects of a finely-characterized environment at the abiotic and biotic level. Combining tree inventories, LiDAR-derived topographic data, leaf functional traits sampling, gene capture experiments, population genomics, environmental association analyses, genome wide association studies and Bayesian modelling for species distribution, functional traits variation, and growth on Symphonia and Eschweilera species complexes, I addressed the following general question:
General: Are individual genotypes and phenotypes adapted to microgeographic abiotic and biotic environments within and among species of species complexes?
I hypothesised individual genetic and phenotypic variability within and among species to promote local coexistence within species complexes through microgeographic adaptations and niche partitioning across topography and forest gap dynamics (Fig. 1). This general hypothesis will be explored all along the thesis with subsequent questions, hypotheses and corresponding chapters.
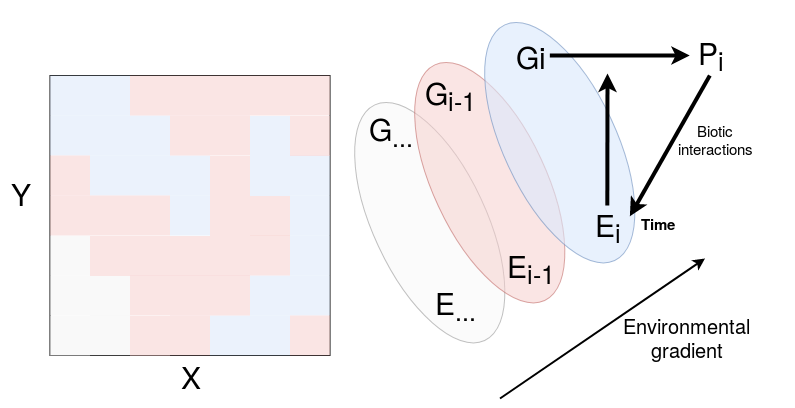
Figure 1: Microgeographic adaptations among sympatric species within a species complex. Different genetic species grow in sympatry in specific habitats along an environmental gradient. The interaction of local environment and genotype result in phenotype . Phenotype feeds back to its local environment through biotic interactions. Temporal variation of the environment influences the phenotype of the established genotype.
To explore genotype-environment interactions, I needed first to characterise abiotic and biotic factors structuring ecological niches within and among species complexes ( in Fig. 1). I addressed the following question:
Chapter 1. How are species distributed within and among species complexes along biotic and abiotic gradients in Paracou?
I hypothesised species complexes to be widely spread across biotic and abiotic environments; whereas biotic and abiotic environments favour niche differentiation among species within species complexes.
To continue in the ecological theater, I needed to explore the role of abiotic and biotic environment on functional traits within and among closely-related species ( to in Fig. 1). I addressed the following question:
Chapter 2. How does the abiotic environment influence individual leaf trait values among and within closely-related species within species complexes?
I hypothesised the abiotic environment to shape trait variation both among and within species in interaction with tree diameter and access to light within species complexes.
Once ecological niches defined and their relations with phenotypes explored, I could dive into individual and species adaptive genomics ( to in Fig. 1). I addressed individual and species adaptation to topography with the following question:
Chapter 3. Are tree species and individuals adapted to the fine-scale abiotic gradient?
I hypothesised species to be delimited along topography with fixed neutral and adaptive variants among and within species of species complexes.
Similarly, I addressed individual and species adaptation to forest gap dynamics with the following question:
Chapter 4. Are tree species and individuals adapted to a trade-off between growth and light access in response to forest gap dynamics?
I hypothesised individual genotypes to be adapted to a trade-off between growth and light access in response to forest gap dynamics.
Finally, once I explored the biotic and abiotic niches of individuals and species within species complexes, the resulting phenotypes and the underlying genomic adaptations, I was able to study the role of genotype-environment interactions in the coexistence of individuals and species within species complexes. I addressed the following question:
Discussion. How do tree species’ and individual’s adaptations to microgeographic topography and forest gap dynamics drive coexistence within and among species of the Symphonia species complex ?
I hypothesised genotypic adaptations within species of Symphonia to decrease the risk of a stochastic local extinction due to wide successional niches which respond to fine spatio-temporal dynamics of forest gaps; and adaptations among species to stabilize local coexistence with differentiated species’ topographic niches within the Symphonia species complex.
References
Aarssen, L. W. 1983. Ecological Combining Ability and Competitive Combining Ability in Plants: Toward a General Evolutionary Theory of Coexistence in Systems of Competition. - The American Naturalist 122: 707–731.
Aguilos, M. et al. 2018. Interannual and Seasonal Variations in Ecosystem Transpiration and Water Use Efficiency in a Tropical Rainforest. - Forests 10: 14.
Albert, C. H. et al. 2010b. Intraspecific functional variability: Extent, structure and sources of variation. - Journal of Ecology 98: 604–613.
Albert, C. H. et al. 2011. When and how should intraspecific variability be considered in trait-based plant ecology? 13: 217–225.
Allié, E. et al. 2015. Pervasive local-scale tree-soil habitat association in a tropical forest community. - PLoS ONE 10: e0141488.
Antonelli, A. and Sanmartín, I. 2011. Why are there so many plant species in the Neotropics? - TAXON 60: 403–414.
Aubry-Kientz, M. et al. 2013. Toward Trait-Based Mortality Models for Tropical Forests (F de Bello, Ed.). - PLoS ONE 8: e63678.
Aubry-Kientz, M. et al. 2015a. A joint individual-based model coupling growth and mortality reveals that tree vigor is a key component of tropical forest dynamics. - Ecology and evolution 5: 2457–65.
Aubry-Kientz, M. et al. 2015b. Identifying climatic drivers of tropical forest dynamics. - Biogeosciences 12: 5583–5596.
Audigeos, D. et al. 2013. Molecular divergence in tropical tree populations occupying environmental mosaics. - Journal of Evolutionary Biology 26: 529–544.
Bailey, J. K. et al. 2009. From genes to ecosystems: A synthesis of the effects of plant genetic factors across levels of organization. - Philosophical Transactions of the Royal Society B: Biological Sciences 364: 1607–1616.
Barabás, G. and D’Andrea, R. 2016. The effect of intraspecific variation and heritability on community pattern and robustness (D Vasseur, Ed.). 19: 977–986.
Baraloto, C. et al. 2005. Performance trade-offs among tropical tree seedlings in contrasting microhabitats. - Ecology 86: 2461–2472.
Baraloto, C. et al. 2007. Seasonal water stress tolerance and habitat associations within four Neotropical tree genera. - Ecology 88: 478–489.
Baraloto, C. et al. 2010a. Decoupled leaf and stem economics in rain forest trees. - Ecology Letters 13: 1338–1347.
Baraloto, C. et al. 2012a. Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities. - Journal of Ecology 100: 690–701.
Barret, R. and Schluter, D. 2008. Adaptation from standing genetic variation. - Trends in Ecology & Evolution 23: 38–44.
Benito Garzón, M. et al. 2019. TraitSDMs: species distribution models that account for local adaptation and phenotypic plasticity. - New Phytologist 222: 1757–1765.
Bonal, D. et al. 2008. Impact of severe dry season on net ecosystem exchange in the Neotropical rainforest of French Guiana. - Global Change Biology 14: 1917–1933.
Borges, R. M. 2009. Phenotypic plasticity and longevity in plants and animals: Cause and effect? 34: 605–611.
Breugel, M. van et al. 2012. The relative importance of above- versus belowground competition for tree growth during early succession of a tropical moist forest. - Plant Ecology 213: 25–34.
Brousseau, L. et al. 2013. Highly local environmental variability promotes intrapopulation divergence of quantitative traits: an example from tropical rain forest trees. - Annals of Botany 112: 1169–1179.
Brousseau, L. et al. 2015. Neutral and Adaptive Drivers of Microgeographic Genetic Divergence within Continuous Populations: The Case of the Neotropical Tree Eperua falcata (Aubl.) (FA Aravanopoulos, Ed.). - PLOS ONE 10: e0121394.
Brown, J. H. et al. 2004. Toward a metabolic theory of ecology. - Ecology 85: 1771–1789.
Bruelheide, H. et al. 2018. Global trait–environment relationships of plant communities. - Nature Ecology & Evolution: 1.
Buchman, R. G. et al. 1983. A tree survival model with application to species of the Great Lakes region. - Canadian Journal of Forest Research 13: 601–608.
Budde, K. B. et al. 2013. The ancient tropical rainforest tree Symphonia globulifera L. f. (Clusiaceae) was not restricted to postulated Pleistocene refugia in Atlantic Equatorial Africa. - Heredity 111: 66–76.
Budde, K. B. et al. 2014. In situ genetic association for serotiny, a fire-related trait, in Mediterranean maritime pine (Pinus pinaster). - New Phytologist 201: 230–241.
Callaway, R. M. et al. 2012. Phenotypic Plasticity and Interactions among Plants. - America 84: 1115–1128.
Canham, C. D. et al. 1990. Light regimes beneath closed canopies and tree-fall gaps in temperate and tropical forests. - Canadian Journal of Forest Research 20: 620–631.
Cannon, C. H. and Lerdau, M. 2015. Variable mating behaviors and the maintenance of tropical biodiversity. - Frontiers in Genetics 6: 183.
Cannon, C. H. and Petit, R. J. 2019. The oak syngameon: more than the sum of its parts. - New Phytologist: nph.16091.
Cardinale, B. J. et al. 2012. Biodiversity loss and its impact on humanity. - Nature 486: 59–67.
Carneiro, F. da S. et al. 2009. High levels of pollen dispersal detected through paternity analysis from a continuous Symphonia globulifera population in the Brazilian Amazon. - Forest Ecology and Management 258: 1260–1266.
Caron, H. et al. 2019. Chloroplast DNA variation in a hyperdiverse tropical tree community. - Ecology and Evolution 9: ece3.5096.
Chave, J. 2004. Neutral theory and community ecology. - Ecology Letters 7: 241–253.
Chave, J. et al. 2009. Towards a worldwide wood economics spectrum. - Ecology Letters 12: 351–366.
Chesson, P. 2000a. Mechanisms of maintenance of species diversity. - Annual review of Ecology and Systematics 31: 343–366.
Chesson, P. 2000b. Mechanisms of Maintenance of Species Diversity. - Annual Review of Ecology and Systematics 31: 343–366.
Clark, J. S. 2010. Individuals and the Variation Needed for High Species Diversity in Forest Trees. - Science 327: 1129–1132.
Clark, D. A. and Clark, D. B. 1984. Spacing Dynamics of a Tropical Rain Forest Tree: Evaluation of the Janzen-Connell Model. - The American Naturalist 124: 769–788.
Comita, L. S. et al. 2010. Asymmetrie density dependence shapes species abundances in a tropical tree community. - Science 329: 330–332.
Connell, J. H. 1978. Diversity in tropical rain forests and coral reefs. - Science 199: 1302–1310.
Craven, D. et al. 2015. Changing gears during succession: shifting functional strategies in young tropical secondary forests. - Oecologia 179: 293–305.
Darwin, C. 1909. The origin of species. - PF Collier & son New York.
Davidson, E. A. et al. 2012. The Amazon basin in transition. 481: 321–328.
Degen, B. et al. 2004. Limited pollen dispersal and biparental inbreeding in Symphonia globulifera in French Guiana. - Heredity 93: 585–591.
Degen, B. et al. 2006. Impact of selective logging on genetic composition and demographic structure of four tropical tree species. - Biological Conservation 131: 386–401.
Delor, C. et al. 2003. Transamazonian crustal growth and reworking as revealed by the 1:500,000-scale geological map of French Guiana (2. - Géologie de la France: 5–57.
De Queiroz, K. 2007. Species concepts and species delimitation. - Systematic Biology 56: 879–886.
Dewoody, J. et al. 2015. Genetic and morphological differentiation in Populus nigra L.: isolation by colonization or isolation by adaptation? - Molecular Ecology 24: 2641–2655.
Dezécache, C. et al. 2017a. Moving forward socio-economically focused models of deforestation. - Global Change Biology 23: 3484–3500.
Dezécache, C. et al. 2017b. Gold-rush in a forested El Dorado: Deforestation leakages and the need for regional cooperation. - Environmental Research Letters in press.
Dick, C. W. and Heuertz, M. 2008. The complex biogeographic history of a widespread tropical tree species. - Evolution 62: 2760–2774.
Dick, C. W. et al. 2004. Molecular Systematic Analysis Reveals Cryptic Tertiary Diversification of a Widespread Tropical Rain Forest Tree. - The American Naturalist 162: 691–703.
Díaz, S. et al. 2016. The global spectrum of plant form and function. - Nature 529: 167–171.
Dobzhansky, T. 1973. Nothing in biology make sense except in the light of evolution. - The american biology teacher 35: 125–129.
Eiserhardt, W. L. et al. 2017. Plant phylogeny as a window on the evolution of hyperdiversity in the tropical rainforest biome. - New Phytologist 214: 1408–1422.
Epron, D. et al. 2006. Spatial variation of soil respiration across a topographic gradient in a tropical rain forest in French Guiana. - Journal of Tropical Ecology 22: 565–574.
Fay, J. C. and Wu, C. I. 2000. Hitchhiking under positive Darwinian selection. - Genetics 155: 1405–1413.
Ferry, B. et al. 2003. Genèse et fonctionnement hydrique des sols sur socle cristallin en Guyane. - Revue Forestiere Francaise 55: 37–56.
Ferry, B. et al. 2010a. Higher treefall rates on slopes and waterlogged soils result in lower stand biomass and productivity in a tropical rain forest. - Journal of Ecology 98: 106–116.
Flood, P. J. and Hancock, A. M. 2017. The genomic basis of adaptation in plants. - Current Opinion in Plant Biology 36: 88–94.
Fortunel, C. et al. 2012. Leaf, stem and root tissue strategies across 758 Neotropical tree species. - Functional Ecology 26: 1153–1161.
Gaggiotti, O. E. and Foll, M. 2010. Quantifying population structure using the F-model. 10: 821–830.
Gaston, K. J. 2000. Global patterns in biodiversity. - Nature 405: 220–227.
Gentry, A. H. 1982. Patterns of Neotropical Plant Species Diversity. - Evolutionary Biology: 1–84.
Gentry, A. H. 1988. Tree species richness of upper Amazonian forests. - Proceedings of the National Academy of Sciences 85: 156–159.
Gonzalez, M. A. et al. 2009. Identification of amazonian trees with DNA barcodes. - PLoS ONE 4: e7483.
Goulamoussène, Y. et al. 2017. Environmental control of natural gap size distribution in tropical forests. - Biogeosciences 14: 353–364.
Gourlet-Fleury, S. et al. 2004. Ecology and management of a neotropical rainforest : lessons drawn from Paracou, a long-term experimental research site in French Guiana Ecology and management of a neotropical rainforest : lessons drawn from Paracou, a long-term experimental research sit. - Paris: Elsevier.
Grant, P. R. and Grant, B. R. 2019. Hybridization increases population variation during adaptive radiation. - Proceedings of the National Academy of Sciences of the United States of America 116: 23216–23224.
Guitet, S. et al. 2013. Landform and landscape mapping, French Guiana (South America). - Journal of Maps 9: 325–335.
Gunatilleke, C. V. S. et al. 2006. Species–habitat associations in a {Sri} {Lankan} dipterocarp forest. - Journal of Tropical Ecology 22: 371.
Hallgrímsson, B. and Hall, B. K. 2005. Variation and variability: Central concepts in biology. - In: Variation. ppp. 1–7.
Hammond, D. S. 2005. Tropical forests of the Guiana Shield: ancient forests in a modern world. - CABI.
Hamrick, J. L. et al. 1992. Factors influencing levels of genetic diversity in woody plant species. - New Forests 6: 95–124.
Hardy, O. J. et al. 2005. Fine-scale genetic structure and gene dispersal inferences in 10 Neotropical tree species. - Molecular Ecology 15: 559–571.
Herault, B. et al. 2010. Growth responses of neotropical trees to logging gaps. - Journal of Applied Ecology 47: 821–831.
Heuertz, M. et al. 2006. Multilocus patterns of nucleotide diversity, linkage disequilibrium and demographic history of Norway spruce [Picea abies (L.) Karst]. - Genetics 174: 2095–2105.
Heuertz, M. et al. 2020. The hyperdominant tropical tree Eschweilera coriacea (Parvifolia clade, Lecythidaceae) shows higher genetic heterogeneity than sympatric Eschweilera species in French Guiana. - Plant Ecology and Evolution. 153: 67–81.
Hérault, B. 2007. Reconciling niche and neutrality through the Emergent Group approach. - Perspectives in Plant Ecology, Evolution and Systematics 9: 71–78.
Hérault, B. and Piponiot, C. 2018. Key drivers of ecosystem recovery after disturbance in a neotropical forest. - Forest Ecosystems 5: 2.
Hérault, B. et al. 2011. Functional traits shape ontogenetic growth trajectories of rain forest tree species. - Journal of Ecology 99: 1431–1440.
Holliday, J. A. et al. 2017. Advances in ecological genomics in forest trees and applications to genetic resources conservation and breeding. - Molecular Ecology: 706–717.
Huang, Y. Y. et al. 2015. Toward a phylogenetic-based generic classification of neotropical lecythidaceae—I. Status of Bertholletia, Corythophora, Eschweilera and Lecythis. - Phytotaxa 203: 085–121.
Hubbell, S. P. 2001. The unified neutral theory of biodervisity.
Hubbell, S. P. et al. 1999. Light-gap disturbances, recruitment limitation, and tree diversity in a neotropical forest. - Science 283: 554–557.
Hulshof, C. M. and Swenson, N. G. 2010b. Variation in leaf functional trait values within and across individuals and species: An example from a Costa Rican dry forest. - Functional Ecology 24: 217–223.
Hutchinson, G. E. 1941. Ecological Aspects of Succession in Natural Populations. - The American Naturalist 75: 406–418.
Hyatt, L. A. et al. 2003. The distance dependence prediction of the Janzen-Connell hypothesis: A meta-analysis. - Oikos 103: 590–602.
John, R. et al. 2007. Soil nutrients influence spatial distributions of tropical tree species. - Proceedings of the National Academy of Sciences of the United States of America 104: 864–9.
Jones, F. A. and Hubbell, S. P. 2006. Demographic spatial genetic structure of the Neotropical tree, Jacaranda copaia. - Molecular Ecology 15: 3205–3217.
Jucker, T. et al. 2018. Topography shapes the structure, composition and function of tropical forest landscapes. - Ecology Letters 21: 989–1000.
Jung, V. et al. 2010a. Intraspecific variability and trait-based community assembly. - Journal of Ecology 98: 1134–1140.
Korte, A. and Farlow, A. 2013. The advantages and limitations of trait analysis with GWAS: A review. 9: 29.
Kraft, N. J. B. et al. 2008. Functional traits and niche-based tree community assembly in an Amazonian forest. - Science 322: 580–582.
Kremer, A. et al. 2012. Long-distance gene flow and adaptation of forest trees to rapid climate change. - Ecology Letters 15: 378–392.
Lascoux, M. et al. 2016. Local Adaptation in Plants. - eLS: 1–7.
Laughlin, D. C. and Laughlin, D. E. 2013. Advances in modeling trait-based plant community assembly. 18: 584–593.
Laughlin, D. C. et al. 2012. A predictive model of community assembly that incorporates intraspecific trait variation (T Fukami, Ed.). - Ecology Letters 15: 1291–1299.
Le Bagousse-Pinguet, Y. et al. 2014. Species richness of limestone grasslands increases with trait overlap: Evidence from within- and between-species functional diversity partitioning (D Wardle, Ed.). - Journal of Ecology 102: 466–474.
Leclerc, T. et al. 2015. Life after disturbance (I): changes in the spatial genetic structure of Jacaranda copaia (Aubl.) D. Don (Bignonianceae) after logging in an intensively studied plot in French Guiana. - Annals of Forest Science 72: 509–516.
Leroy, T. et al. 2019. Massive postglacial gene flow between European white oaks uncovered genes underlying species barriers. - New Phytologist in press.
Levi, T. et al. 2019a. Tropical forests can maintain hyperdiversity because of enemies. - Proceedings of the National Academy of Sciences 116: 581–586.
Lewis, S. L. et al. 2004. Fingerprinting the impacts of global change on tropical forests. - Philosophical Transactions: Biological Sciences 359: 437–462.
Lewis, S. L. et al. 2015. Increasing human dominance of tropical forests. 349: 827–832.
Lichstein, J. W. et al. 2007. Intraspecific Variation and Species Coexistence. - The American Naturalist 170: 807–818.
Linnaeus, C. 1938. The "Critica Botanica" of Linnæus. - Nature 141: 993–994.
Lloyd, J. et al. 2013. Photosynthetically relevant foliar traits correlating better on a mass vs an area basis: of ecophysiological relevance or just a case of mathematical imperatives and statistical quicksand? - New Phytologist 199: 311–321.
Lortie, C. J. et al. 2004a. Rethinking plant community theory. - Oikos 107: 433–438.
Mayden, R. L. 1997. A hierarchy of species concepts: the denouement in the saga of the species problem. - Species. The units of biodiversity.: 381–423.
Mayr, E. 1996. What Is a Species, and What Is Not? - Philosophy of Science 63: 262.
Mcgill, B. et al. 2006. Rebuilding community ecology from functional traits. - Trends in Ecology & Evolution 21: 178–185.
Messer, P. W. and Petrov, D. A. 2013. Population genomics of rapid adaptation by soft selective sweeps. - Trends in Ecology and Evolution 28: 659–669.
Messier, J. et al. 2010a. How do traits vary across ecological scales? A case for trait-based ecology. - Ecology Letters 13: 838–848.
Messier, J. et al. 2010b. How do traits vary across ecological scales? A case for trait-based ecology. - Ecology Letters 13: 838–848.
Molino, J.-F. and Sabatier, D. 2001. Tree Diversity in Tropical Rain Forests: A Validation of the Intermediate Disturbance Hypothesis. - Science 294: 1702–1704.
Mori, S. A. et al. 2016. Observations on the phytogeography of the LECYTHIDACEAE clade (Brazil nut family). 30: 1–85.
Negrón-Juárez, R. I. et al. 2018. Vulnerability of Amazon forests to storm-driven tree mortality. - Environmental Research Letters 13: 054021.
Niklas, K. J. and Enquist, B. J. 2003. An allometric model for seed plant reproduction. - Evolutionary Ecology Research 5: 79–88.
Nybom, H. 2004. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. - Molecular Ecology 13: 1143–1155.
Odonne, G. et al. 2019. Long-term influence of early human occupations on current forests of the Guiana Shield. - Ecology: e02806.
Olsson, S. et al. 2017. Development of genomic tools in a widespread tropical tree, Symphonia globulifera L.f.: a new low-coverage draft genome, SNP and SSR markers. - Molecular Ecology Resources 17: 614–630.
Osazuwa-Peters, O. L. et al. 2017. Linking wood traits to vital rates in tropical rainforest trees: Insights from comparing sapling and adult wood. - American Journal of Botany 104: 1464–1473.
Osnas, J. L. D. et al. 2013. Global Leaf Trait Relationships: Mass, Area, and the Leaf Economics Spectrum. - Science 340: 741–744.
Paine, C. E. T. et al. 2011. Functional traits of individual trees reveal ecological constraints on community assembly in tropical rain forests. - Oikos 120: 720–727.
Paun, O. et al. 2016. Processes Driving the Adaptive Radiation of a Tropical Tree (Diospyros, Ebenaceae) in New Caledonia, a Biodiversity Hotspot. - Systematic Biology 65: 212–227.
Pearson, T. R. H. et al. 2017. Greenhouse gas emissions from tropical forest degradation: an underestimated source. - Carbon Balance and Management 12: 3.
Pelletier, F. et al. 2009. Eco-evolutionary dynamics. - Philosophical Transactions of the Royal Society B: Biological Sciences 364: 1483–1489.
Pernès, J. and Lourd, M. 1984. Organisation des complexes d’espèces. - Gestion de ressources genetiques des plantes in press.
Perrier de la Bâthie, H. 1951. Guttiferes. - In: Flore de madagascar et des comores (plantes vasculaires). H. Humbert. Firmin-Didot, ppp. 1–186.
Petit, R. J. and Hampe, A. 2006. Some Evolutionary Consequences of Being a Tree. - Annual Review of Ecology, Evolution, and Systematics 37: 187–214.
Petit, R. J. et al. 2002. Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. - Forest Ecology and Management 156: 49–74.
Petit, R. J. et al. 2004. Hybridization as a mechanism of invasion in oaks. 161: 151–164.
Philipson, C. D. et al. 2014. A trait-based trade-off between growth and mortality: Evidence from 15 tropical tree species using size-specific relative growth rates. - Ecology and Evolution 4: 3675–3688.
Pillon, Y. et al. 2014. Cryptic adaptive radiation in tropical forest trees in New Caledonia. - New Phytologist 202: 521–530.
Pinheiro, F. et al. 2018. Plant Species Complexes as Models to Understand Speciation and Evolution: A Review of South American Studies. - Critical Reviews in Plant Sciences 37: 54–80.
Piponiot, C. et al. 2016. Carbon recovery dynamics following disturbance by selective logging in Amazonian forests. - eLife 5: e21394.
Pitman, N. C. et al. 2001. Dominance and distribution of tree species in upper Amazonian terra firme forests. - Ecology 82: 2101–2117.
Plomion, C. et al. 2018. Oak genome reveals facets of long lifespan. - Nature Plants 4: 440–452.
Poorter, L. and Bongers, F. 2006. Leaf traits are good predictors of plant performance across 53 raun forest species. - Ecology 87: 1733–1743.
Reich, P. B. 2014a. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. - Journal of Ecology 102: 275–301.
Richardson, J. L. et al. 2014. Microgeographic adaptation and the spatial scale of evolution. - Trends in Ecology and Evolution 29: 165–176.
Román-Dañobeytia, F. J. et al. 2012. Testing the Performance of Fourteen Native Tropical Tree Species in Two Abandoned Pastures of the Lacandon Rainforest Region of Chiapas, Mexico. - Restoration Ecology 20: 378–386.
Rundell, R. J. and Price, T. D. 2009. Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. - Trends in Ecology & Evolution 24: 394–399.
Runemark, A. et al. 2019. Eukaryote hybrid genomes. - PLOS Genetics 15: e1008404.
Sabeti, P. C. et al. 2002. Detecting recent positive selection in the human genome from haplotype structure. - Nature 419: 832–837.
Salguero-Gómez, R. 2017. Applications of the fast–slow continuum and reproductive strategy framework of plant life histories. - New Phytologist 213: 1618–1624.
Salguero-Gómez, R. et al. 2018. Delivering the promises of trait-based approaches to the needs of demographic approaches, and vice versa. - Functional Ecology 32: 1424–1435.
Savolainen, V. et al. 2006. Sympatric speciation in palms on an oceanic island. - Nature 441: 210–213.
Savolainen, O. et al. 2007. Gene Flow and Local Adaptation in Trees. - Annual Review of Ecology, Evolution, and Systematics 38: 595–619.
Savolainen, O. et al. 2013. Ecological genomics of local adaptation. - Nature Reviews Genetics 14: 807–820.
Scheffer, M. and Van Nes, E. H. 2006. Self-organized similarity, the evolutionary emergence of groups of similar species. - Proceedings of the National Academy of Sciences of the United States of America 103: 6230–6235.
Schmitt, S. et al. 2019. Functional diversity improves tropical forest resilience: insights from a long-term virtual experiment. - Journal of Ecology: 1365–2745.13320.
Scotti, I. et al. 2015. Life after disturbance (II): the intermediate disturbance hypothesis explains genetic variation in forest gaps dominated by Virola michelii Heckel (Myristicaceae). - Annals of Forest Science 72: 1035–1042.
Scotti, I. et al. 2016. Fifty years of genetic studies: what to make of the large amounts of variation found within populations? - Annals of Forest Science 73: 69–75.
Seehausen, O. 2004. Hybridization and adaptive radiation. - Trends in Ecology & Evolution 19: 198–207.
Siefert, A. and Ritchie, M. E. 2016. Intraspecific trait variation drives functional responses of old-field plant communities to nutrient enrichment. - Oecologia 181: 245–255.
Siefert, A. et al. 2015a. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. - Ecology Letters 18: 1406–1419.
Siefert, A. et al. 2015b. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. - Ecology Letters 18: 1406–1419.
Smith, J. M. and Haigh, J. 1974. The hitch-hiking effect of a favourable gene. - Genetical Research 23: 23–35.
Soininen, J. 2010. Species Turnover along Abiotic and Biotic Gradients: Patterns in Space Equal Patterns in Time? - BioScience 60: 433–439.
Soong, J. L. et al. 2020. Soil properties explain tree growth and mortality, but not biomass, across phosphorus-depleted tropical forests. - Scientific Reports 10: 1–13.
Steege, H. ter et al. 2006. Continental-scale patterns of canopy tree composition and function across {Amazonia}. - Nature 443: 444–447.
Steege, H. ter et al. 2013. Hyperdominance in the Amazonian Tree Flora. - Science 342: 1243092–1243092.
Suarez-Gonzalez, A. et al. 2018. Adaptive introgression: a plant perspective. - Biology Letters 14: 20170688.
Sultan, S. E. 1987. Evolutionary implications of phenotypic plasticity in plants. - Evolutionary Biology: 127–178.
Thomson, A. M. et al. 2018. Complete plastome sequences from Bertholletia excelsa and 23 related species yield informative markers for Lecythidaceae. - Applications in Plant Sciences 6: e01151.
Tigano, A. and Friesen, V. L. 2016. Genomics of local adaptation with gene flow. - Molecular Ecology 25: 2144–2164.
Tobias, J. A. et al. 2014. Species coexistence and the dynamics of phenotypic evolution in adaptive radiation. - Nature 506: 359–363.
Torroba-Balmori, P. et al. 2017. Altitudinal gradients, biogeographic history and microhabitat adaptation affect fine-scale spatial genetic structure in African and Neotropical populations of an ancient tropical tree species. - PLOS ONE 12: e0182515.
Turcotte, M. M. and Levine, J. M. 2016. Phenotypic Plasticity and Species Coexistence. - Trends in Ecology & Evolution 31: 803–813.
Tutt, J. W. 1896. British moths. - G. Routledge.
Unger, G. M. et al. 2016. Assessing early fitness consequences of exotic gene flow in the wild: A field study with Iberian pine relicts. - Evolutionary Applications 9: 367–380.
Vargas, O. M. et al. 2019. Target sequence capture in the Brazil nut family (Lecythidaceae): Marker selection and in silico capture from genome skimming data. - Molecular Phylogenetics and Evolution 135: 98–104.
Vellend, M. 2006. The consequences of genetic diversity in competitive communities. - Ecology 87: 304–311.
Vieilledent, G. et al. 2010. Individual variability in tree allometry determines light resource allocation in forest ecosystems: A hierarchical Bayesian approach. - Oecologia 163: 759–773.
Vincent, G. et al. 2012. Accuracy of small footprint airborne LiDAR in its predictions of tropical moist forest stand structure. - Remote Sensing of Environment 125: 23–33.
Violle, C. et al. 2007. Let the concept of trait be functional! - Oikos 116: 882–892.
Violle, C. et al. 2012. The return of the variance: Intraspecific variability in community ecology. - Trends in Ecology and Evolution 27: 244–252.
Volkov, I. et al. 2005. Density dependence explains tree species abundance and diversity in tropical forests. - Nature 438: 658–661.
Wagner, F. et al. 2011. Modeling water availability for trees in tropical forests. - Agricultural and Forest Meteorology 151: 1202–1213.
Wagner, F. et al. 2012. Water availability is the main climate driver of neotropical tree growth (G Bohrer, Ed.). - PLoS ONE 7: e34074.
Weiher, E. and Keddy, P. A. 1995. Assembly rules, null models, and trait dispersion: new questions from old patterns. - Oikos 74: 159–164.
Whitlock, R. et al. 2007. The role of genotypic diversity in determining grassland community structure under constant environmental conditions. - Journal of Ecology 95: 895–907.
Whitney, K. D. et al. 2010. Patterns of hybridization in plants. - Perspectives in Plant Ecology, Evolution and Systematics 12: 175–182.
Wiens, J. J. et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. - Ecology Letters 13: 1310–1324.
Wilson, A. J. et al. 2010. An ecologist’s guide to the animal model. - Journal of Animal Ecology 79: 13–26.
Wright, J. S. 2002. Plant diversity in tropical forests: a review of mechanisms of species coexistence. - Oecologia 130: 1–14.
Wright, I. J. and Westoby, M. 2002. Leaves at low versus high rainfall: Coordination of structure, lifespan and physiology. - New Phytologist 155: 403–416.
Wright, I. J. et al. 2004. The worldwide leaf economics spectrum. - Nature 428: 821–827.
Wright, S. J. et al. 2010. Functional traits and the growth-mortality trade-off in tropical trees. - Ecology 91: 3664–3674.
Yamasaki, N. et al. 2013. Coexistence of two congeneric tree species of Lauraceae in a secondary warm-temperate forest on Miyajima Island, south-western Japan. - Plant Species Biology 28: 41–50.
Zeng, J. et al. 2018. Signatures of negative selection in the genetic architecture of human complex traits. - Nature Genetics 50: 746–753.
Zhou, X. and Stephens, M. 2012. Genome-wide efficient mixed-model analysis for association studies. - Nature Genetics 44: 821–824.